Saudi Arabia Bio-Implant Market Analysis
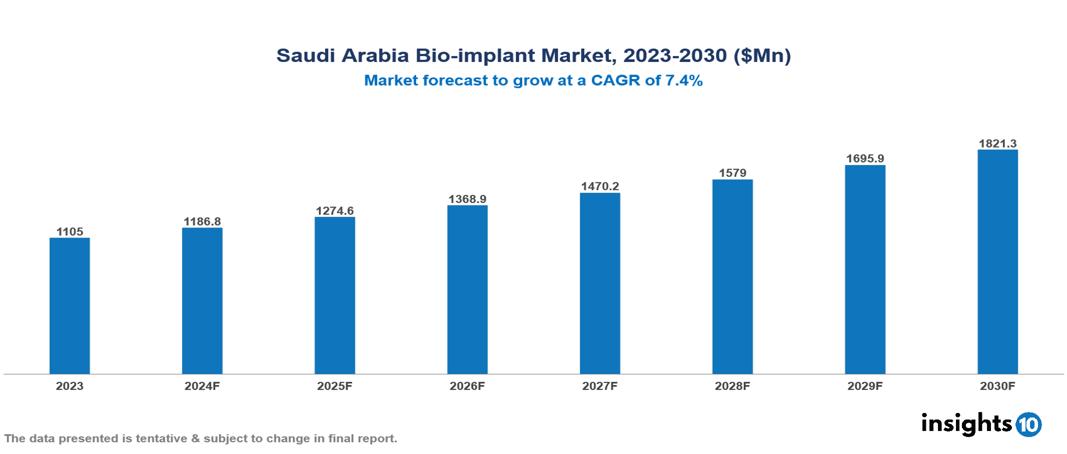
The Saudi Arabia Bio-implant Market was valued at $1105 Mn in 2023 and is predicted to grow at a CAGR of 7.4% from 2023 to 2030, to $1821.3 Mn by 2030. The Saudi Arabia Bio-implant Market is growing due to Rising Medical Tourism, Growing Awareness and Acceptance, and Government Healthcare Initiatives. The market is primarily dominated by players such as Cochlear Ltd. Edwards Lifesciences Corporation, Berlin Heart, Medtronic, Jarvik Heart Inc., Abiomed, Zimmer Biomet, Boston Scientific Corporation, Otto Bock Holding GmbH & Co. KG, and Medtronic plc.
Buy Now

Saudi Arabia Bio-implant Market Executive Summary
The Saudi Arabia Bio-implant Market is at around $1105 Mn in 2023 and is projected to reach $1821.3 Mn in 2030, exhibiting a CAGR of 7.4% during the forecast period.
Bioimplants are advanced medical devices designed to be implanted into the body in order to support or replace a biological structure or function. These instruments range significantly in complexity and purpose from simple dental implants to sophisticated gadgets like pacemakers, prosthetic joints, and neurological implants. One of the primary objectives of bioimplants is to enhance the quality of life for people with a range of diseases or injuries by using implantable devices to restore lost or damaged functions. Bio-implants, which are often composed of biocompatible materials, reduce the likelihood of rejection. Innovations like adding wireless connectivity and sensors to bioimplants, which enable real-time physiological data monitoring and remote modifications, are driving the market's growth.
In Saudi Arabia, chronic diseases like diabetes and cardiovascular conditions affect a significant portion of the population, driven by lifestyle changes and urbanization. The country's aging population is increasing, projected to reach 20% by 2030, influencing demand for orthopedic and dental bioimplants. Demographically, a youthful population with increasing healthcare awareness contributes to market growth, supported by government initiatives promoting healthcare infrastructure development. Therefore, the market is driven by significant factors like Rising Medical Tourism, Growing Awareness and Acceptance, and Government Healthcare Initiatives. However, the High Cost of Bioimplants, Regulatory Hurdles, and Insurance and Reimbursement Issues restrict the growth and potential of the market.
Edwards Lifesciences is a Saudi Arabian company that specializes in heart valve technologies and offers sophisticated heart valve treatments to cardiac patients.
Market Dynamics
Market Growth Drivers
Rising Medical Tourism: Saudi Arabia is positioning itself as a medical tourism hub in the Middle East, attracting patients seeking high-quality medical treatments, including bioimplants. The Kingdom's efforts to enhance healthcare quality and infrastructure are expected to boost medical tourism, projected to reach 500,000 medical tourists by 2030.
Growing Awareness and Acceptance: There is an increasing awareness and acceptance of bioimplants among healthcare providers and patients in Saudi Arabia. Educational campaigns and better information dissemination about the benefits and safety of bioimplants contribute to their growing adoption. A survey conducted by the Saudi Health Council in 2020 indicated that 65% of respondents were open to considering bioimplants for medical treatments.
Government Healthcare Initiatives: The Saudi government's focus on improving healthcare infrastructure and services under the Vision 2030 initiative is a crucial driver. The initiative includes increasing healthcare expenditure, which reached $53.7 Bn in 2022, aimed at enhancing medical facilities and access to advanced treatments, including bioimplants.
Market Restraints
High Cost of Bioimplants: The prohibitive cost of bioimplants remains a significant barrier to their widespread adoption. High expenses associated with the procurement, surgical implantation, and post-operative care of bioimplants limit their accessibility to a broader population. For instance, the cost of orthopedic implants can range between $2,000 to $10,000, a figure that is unaffordable for many middle-class families without substantial insurance coverage.
Regulatory Hurdles: The bioimplant market is subject to stringent regulatory requirements, which can delay the introduction of new products. Companies must navigate a complex approval process involving multiple regulatory bodies. The Saudi Food and Drug Authority (SFDA) requires extensive clinical trials and documentation, which can extend the time-to-market by 1-2 years, thereby discouraging innovation and entry of new players.
Insurance and Reimbursement Issues: The reimbursement policies for bioimplant procedures are often inadequate, leading to high out-of-pocket expenses for patients. The Council of Cooperative Health Insurance (CCHI) has stringent criteria for covering bioimplant surgeries, resulting in limited coverage. For example, less than 50% of bioimplant procedures receive full insurance reimbursement, placing a financial burden on patients.
Regulatory Landscape and Reimbursement Scenario
Saudi Food and Drug Authority (SFDA) ensures the safety, efficacy, and quality of medical devices, including bioimplants. Regulatory requirements include rigorous pre-market approval processes, where manufacturers must provide comprehensive clinical data and evidence of compliance with international standards. The SFDA also mandates post-market surveillance to monitor the performance of bioimplants and address any safety concerns. Saudi Arabia's Vision 2030 initiative aims to enhance the healthcare sector, promoting innovation and investment in medical technologies, including bioimplants.
In Saudi Arabia, the bioimplant market faces reimbursement challenges due to evolving healthcare policies and a preference for conservative treatment approaches. Limited insurance coverage and high out-of-pocket costs for advanced implant procedures hinder market growth. Despite increasing healthcare expenditure and government initiatives to improve access, stringent regulatory frameworks and a reliance on traditional healthcare models contribute to slow adoption rates. Addressing these barriers requires collaborative efforts among stakeholders to enhance reimbursement policies and educate healthcare providers on the long-term benefits of bioimplants, particularly in orthopedics and dental applications.
Competitive Landscape
Key Players
Here are some of the major key players in the Saudi Arabia Bio-implant Market:
- Cochlear Ltd
- Edwards Lifesciences Corporation
- Berlin Heart
- Medtronic
- Jarvik Heart, Inc
- Abiomed
- Straumann AG
- Zimmer Biommer
- Boston Scientific Corporation
- Otto Bock Holding GmbH & Co. KG
- Medtronic
- Boston Scientific Corporation
- Johnson & Johnson Services, Inc.
- LifeNet Health
- Smith & Nephew
- Arthrex, Inc
- Clinic Lemanic
- DePuy Synthes
- Exactech, Inc.
- Cochlear Ltd
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Saudi Arabia Bio-implant Market Segmentation
By Material
- Ceramics
- Polymers
- Alloys
- Biomaterials Metals
By Type
- Dental Bio-implants
- Orthopedic Bio-implants
- Spinal Bio-implants
- Ophthalmology Bio-implants
- Cardiovascular Bio-implants
- Others
By Mode of Administration
- Surgical
- Injectable
By End User
- Hospitals
- Speciality Clinics
- Ambulatory surgical centers
By Origin
- Autograft
- Allograft
- Xenograft
- Synthetic
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































