Saudi Arabia Axial Spondyloarthritis (axSpA) Market Analysis
Saudi Arabia Axial Spondyloarthritis (axSpA) Market is projected to grow from $xx Mn in 2022 to $xx Mn by 2030, registering a CAGR of xx% during the forecast period of 2022 - 2030. With ongoing research and development efforts, the expansion of treatment options, and increasing patient awareness, the axSpA market is expected to witness significant growth in the coming years. AbbVie Inc., Amgen Inc., Johnson & Johnson, Novartis International AG, Pfizer Inc., Eli Lilly and Company, Merck & Co., UCB S.A., Bristol-Myers Squibb Company, Boehringer Ingelheim International GmbH are the global key players in axSpA market.
Buy Now

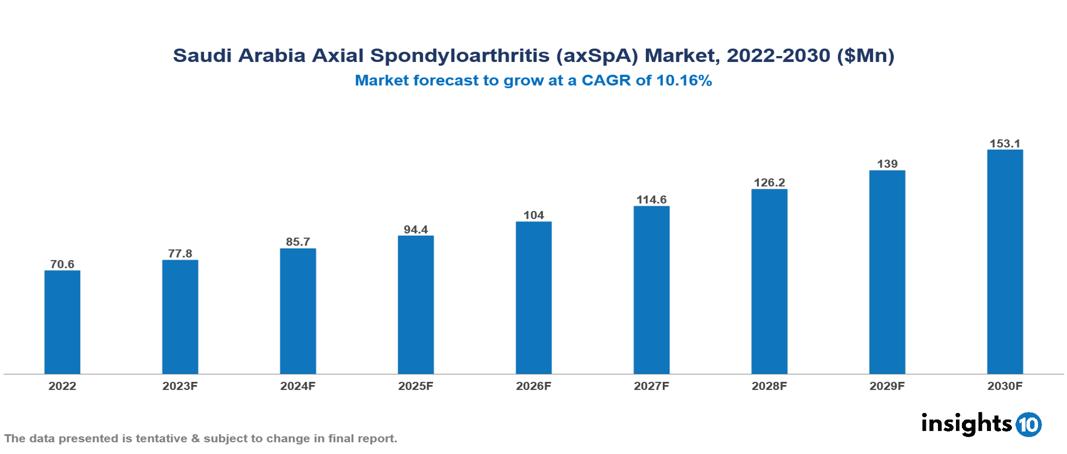
Saudi Arabia Axial Spondyloarthritis (axSpA) Market Analysis Summary
Saudi Arabia Axial Spondyloarthritis (axSpA) Market is valued at around $70.6 Mn in 2022 and is projected to reach $153.1 Mn by 2030, exhibiting a CAGR of 10.16% during the forecast period 2023-2030.
Axial Spondyloarthritis (axSpA) refers to a chronic inflammatory condition primarily affecting the axial skeleton, including the spine and sacroiliac joints. Treatment for Axial Spondyloarthritis (axSpA) typically involves a combination of medication, physical therapy, and lifestyle modifications. Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly prescribed to reduce pain and inflammation. In more severe cases, biological therapies such as tumor necrosis factor (TNF) inhibitors may be used to target the underlying immune response. Regular exercise and maintaining a healthy lifestyle are also important for managing symptoms and improving overall well-being. With ongoing research and development efforts, the expansion of treatment options, and increasing patient awareness, the axSpA market is expected to witness significant growth in the coming years. AbbVie Inc., Amgen Inc., Johnson & Johnson, Novartis International AG, Pfizer Inc., Eli Lilly and Company, Merck & Co., UCB S.A., Bristol-Myers Squibb Company, Boehringer Ingelheim International GmbH are the global key players in axSpA market.
Market Dynamics
Market Growth Drivers
Several significant elements fueling axSpA's growth are highlighted in the company's market analysis. To begin, the rising prevalence of axSpA, combined with more awareness and better diagnostic tools, has resulted in earlier diagnosis and treatment commencement, positively benefiting the market. Second, advances in therapeutic options, particularly the development of biologic medicines targeting specific inflammatory pathways, have transformed axSpA therapy, significantly enhancing patients' quality of life. Furthermore, the increased emphasis on customized care and tailored treatment techniques, as well as the availability of novel medicines, has aided market expansion. Furthermore, collaborations between pharmaceutical corporations and academic institutions, as well as increasing clinical trial investments, have hastened the development of innovative axSpA medicines.
Market Restraints
High Treatment Costs: Biologic therapy and targeted treatments used to treat axSpA can be costly. The high cost of these medications can make them inaccessible to some patients, especially those without adequate insurance coverage or financial resources.
Side Effects and Safety Concerns: AxSpA treatments, like any medical treatment, might have potential side effects and safety concerns. This can include an increased risk of infection, immunosuppression, or severe drug effects. Patients' and healthcare providers' decision-making processes might be influenced by safety concerns and monitoring requirements.
Disease Monitoring Difficulties: AxSpA necessitates regular disease monitoring and assessment of disease activity and progression. However, precisely detecting disease activity, interpreting imaging results, and analyzing treatment response can be difficult. Improved and uniform disease monitoring methods might aid in better patient care.
Lack of Awareness and Education: There is a need for enhanced awareness and education regarding axSpA among healthcare professionals, patients, and the general public. Better knowledge can lead to earlier disease detection, better disease management, and easier access to appropriate therapies.
Competitive Landscape
Key Players
- AbbVie Inc.
- Amgen Inc.
- Johnson & Johnson
- Novartis International AG
- Pfizer Inc.
- Eli Lilly and Company
- Merck & Co.
- UCB S.A.
- Bristol-Myers Squibb Company
- Boehringer Ingelheim International GmbH
Development in Axial Spondyloarthritis (axSpA) Market Analysis
Galapagos NV is conducting a clinical study to evaluate the effect of erlotinib in participants with active axial spondyloarthritis (olinguito). The study is under Phase III evaluation.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Saudi Arabia Axial Spondyloarthritis (axSpA) Market Segmentation
By Therapy
- Anti-tumour Necrosis Factor (TNF) Therapy
- Anti-interleukin (IL) Therapy
- Anti-Janus Kinase (JAK) Therapy
By Indication
- Ankylosing Spondylitis
- Non-radiographic Axial Spondyloarthritis
By End-user
- Hospitals and Clinics
- Academic and Research Institutions
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































