Saudi Arabia Allergy Therapeutics Market Analysis
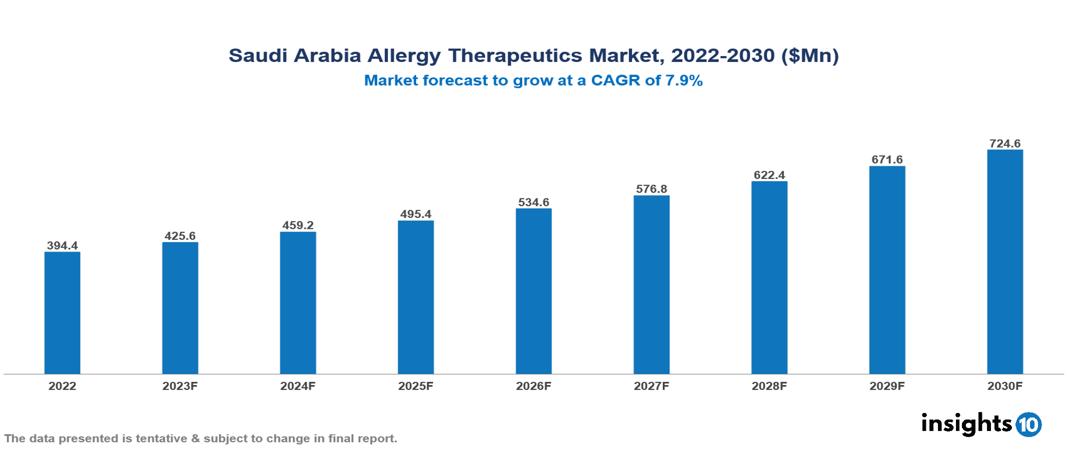
Saudi Arabia allergy therapeutics market was valued at $394 Mn in 2022 and is estimated to reach $725 Mn in 2030, exhibiting a CAGR of 7.9% during the forecast period. The market for allergy therapeutics is expanding as a result of the rising incidence of allergic illnesses and associated medical conditions. Sanofi, Novartis, GlaxoSmithKline (GSK), AstraZeneca, Merck & Co., Johnson & Johnson, Roche, Teva Pharmaceutical Industries, ALK-Abelló, and Stallergenes Greer are few leading companies working in the allergy therapeutics market.
Buy Now

Saudi Arabia Allergy Therapeutics Market Executive Summary
Saudi Arabia allergy therapeutics market was valued at $394 Mn in 2022 and is estimated to reach $725 Mn in 2030, exhibiting a CAGR of 7.9% during the forecast period.
The immune system's sensitivity to harmless environmental substances, or allergens, causes allergies. These allergens can include common dietary triggers like milk, eggs, soy, and almonds, as well as dust mites, pollen, and mold. Allergic reactions ranging from mild to severe can cause symptoms such as stinging eyes and runny noses, while anaphylaxis, a severe reaction, can induce coma and difficulty breathing. Strategies like immunotherapy, avoiding allergens, and using prescription medications like nasal corticosteroids and antihistamines can all be used to control allergies.
In Saudi Arabia, allergies are becoming more and more common, affecting a significant percentage of people of all ages. According to estimates, between 30% and 40% of Saudis suffer from allergies, which is a significant increase. Particular allergies are noteworthy; among the most common is allergic rhinitis, which affects around 35%–40% of people and causes symptoms like congestion, runny noses, itchy eyes, and sneezing, which severely lowers quality of life. 7%–10% of children and 3%–4% of adults suffer from food allergies, which can cause severe responses and require emergency medical intervention. 5%–10% of people have skin allergies, mostly eczema, and 8% of Saudis have lung allergies, including asthma, which are brought on by allergens like mold, dust mites, and pollen. Allergies are becoming more common due to a combination of genetic, environmental, and lifestyle factors.
Research projects aimed at understanding the prevalence and triggers of allergies are actively supported by the King Abdulaziz Science and Technology City (KACST). With the ultimate goal of developing treatment plans that are appropriate for Saudi Arabian culture, the focus is on promoting a greater understanding of the country. Pharmaceutical companies like Al-Hayat Pharmaceutical Company and Tabuk Pharmaceutical Manufacturing Company are making major contributions to the development of allergy therapies within the local pharmaceutical scene. These organizations are allocating funds to research and development projects with the goal of producing allergy treatments, including generic versions of already-approved pharmaceuticals.
Market Dynamics
Market Growth Drivers
Changing Lifestyles: An increase in allergies can be attributed to changes in the environment, urbanization, and lifestyle, which increase the demand for allergy treatment drugs. An increased frequency of allergic disorders may result from variables such as pollution, dietary modifications, and allergen exposure.
Increased Awareness and Diagnosis: People are more likely to seek treatment when allergies are identified sooner and with greater precision, thanks to improved diagnostic capabilities and public awareness campaigns. Due to the rising demand for allergy treatment medications brought on by this greater awareness, the market is expanding.
Increasing Prevalence of Allergies: The need for allergy treatment medications is mostly driven by the increased prevalence of allergic conditions among citizens, including food allergies, skin allergies, and respiratory allergies.
Market Restraints
Regulatory Hurdles: If the licensing process for innovative medications and therapies is delayed, patients might not have timely access to innovative treatments because of the stringent regulatory protocols that need to be followed. This delay in licensing process can hinder the growth of the market.
Treatment Compliance and Adherence: Consistent adherence is essential for allergy therapies to be effective, particularly long-term regimens like immunotherapy. Ineffective patient adherence caused by costs, difficulties, or side effects may lessen therapy efficacy and obstruct market growth.
High Cost of Treatment: A considerable percentage of the population cannot afford many of the modern and successful allergy medicines, such as biologics and sublingual immunotherapy, due to their high cost. This may restrict patients access to the best possible care and market penetration.
Healthcare Policies and Regulatory Landscape
The Saudi Food and Drug Authority (SFDA), the main regulatory organization in charge of ensuring the efficacy, safety, and quality of pharmaceuticals, is in charge of regulating the legal framework for allergy treatment medications in Saudi Arabia. Approval from the SFDA, which oversees drug registration and safety standard compliance, is required for companies looking to market allergy therapy products. The pricing approval procedure is also influenced by the SFDA, which has an effect on the accessibility and affordability of allergy therapy drugs. The SFDA must approve clinical studies for novel medications, including allergy therapies, in order to guarantee ethical and safety requirements. Pharmaceutical product quality standards could be set by the Saudi Arabian Standard Organization (SASO), which would add to the regulatory environment as a whole. The environment for the import, sale, and distribution of medications used to treat allergies is further shaped by rules governing pharmacy practices, customs, and government health policy.
Competitive Landscape
Key Players
- Sanofi
- Novartis
- GlaxoSmithKline (GSK)
- AstraZeneca
- Merck & Co.
- Johnson & Johnson
- Roche
- Teva Pharmaceutical Industries
- ALK-Abelló
- Stallergenes Greer
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Saudi Arabia Allergy Therapeutics Market Segmentation
By Treatment Type
- Anti-allergy drugs
- Immunotherapy
By Type of Allergy
- Eye allergy
- Asthma
- Skin allergy
- Food allergies
- Rhinitis
- Other allergy types
By Route of Administration
- Oral
- Inhalers
- Intranasal
- Other routes of administration
By Distribution Channel
- Hospital pharmacies
- Retail pharmacies
- Online pharmacies
- Other distribution channel
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.