Saudi Arabia Age-Related Macular Degeneration Therapeutics Market Analysis
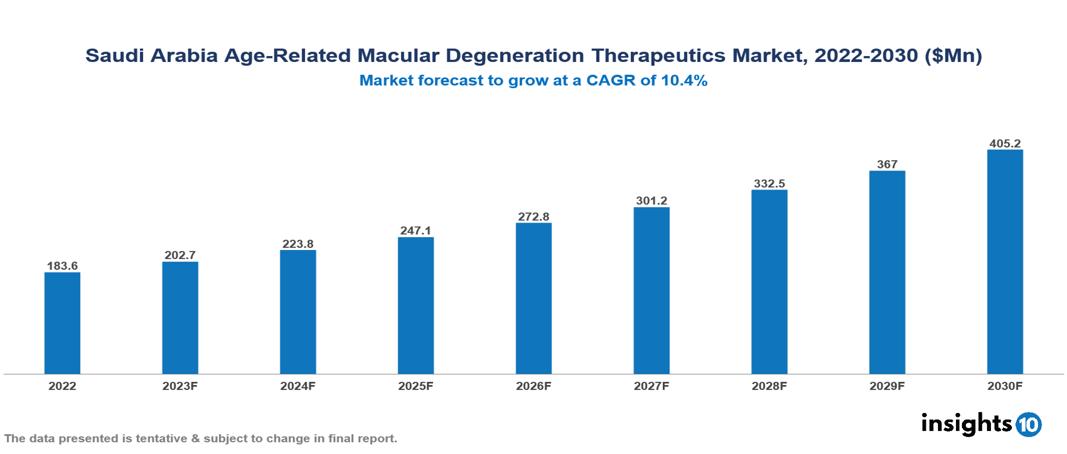
The Saudi Arabia Age-Related Macular Degeneration (AMD) Therapeutics Market was valued at US $184 Mn in 2022, and is predicted to grow at a CAGR of 10.4% from 2023 to 2030, to US $405 Mn by 2030. The key drivers of this industry include the upward trend in the prevalence of age-related macular degeneration, technological advancements, and other factors. The industry is primarily dominated by players such as Regeneron Pharmaceuticals, Novartis, Bayer, Roche, and Genetech, among others
Buy Now

Saudi Arabia Age-Related Macular Degeneration Therapeutics Market Analysis: Executive Summary
The Saudi Arabia Age-Related Macular Degeneration (AMD) Therapeutics Market is at around US $184 Mn in 2022 and is projected to reach US $405 Mn in 2030, exhibiting a CAGR of 10.4% during the forecast period.
Age-related macular degeneration (AMD) is a gradual eye condition that predominantly affects elderly individuals, potentially resulting in significant vision impairment. It primarily impacts the macula, responsible for central vision, making activities such as reading challenging. AMD manifests in two main forms, namely dry AMD and wet AMD. Symptoms include distorted vision, difficulty seeing clearly, and a gradual decline in vision. Dry AMD is more prevalent yet slower in progression. On the other hand, wet AMD, which is less common but more severe, is characterized by abnormal blood vessel growth beneath the macula. Treatment options encompass anti-VEGF therapy, including medications like Lucentis, Vabysmo, Avastin manufactured by Genentech, and Eylea by Regeneron Pharmaceuticals, along with laser therapy and nutritional supplements such as zinc and copper.
It is estimated that the prevalence of AMD is around 4% for the age group of 40 years and older in Saudi Arabia. This prevalence is directly proportional to the age of the population. The market is propelled by key factors such as the growth of the aging population and the consequent rise in prevalence, technological advancements in developing new therapies, increased government initiatives, and consumer knowledge of the therapeutics industry. However, adverse drug reactions and the high expense of therapies like gene therapy and laser surgery, which are adopted in response to demand, limit the market's potential.
The market leader in Saudi Arabia is Regeneron Pharmaceuticals with Eylea, which is an anti-VEGF treatment for wet AMD and earns a market share of approximately 40%. Regeneron is closely followed by its close competitor, Novartis which has a share of around 30%
Market Dynamics
Market Growth Drivers
Demographic shift: The population of Saudi Arabia is ageing disproportionately as compared to its younger age group. The prevalence of AMD in the population aged 40 years and older is around 4%. The increasing prevalence is associated with risk factors like age, smoking, and diabetes, which are anticipated to steadily grow in the forecasted years.
Technological advancements: Progress in diagnostic modalities such as optical coherence tomography (OCT) aids in the prompt and accurate identification of AMD, allowing for the timely commencement of treatment. The landscape for AMD treatments is progressing, with encouraging advancements like gene therapy and long-acting anti-VEGF medications becoming available in the Saudi market, driving growth.
Government initiatives: The healthcare agenda of Saudi Vision 2030 places emphasis on enhancing eye health and expanding access to ophthalmic services, potentially fueling growth in the AMD therapeutics market. The increased allocation of funds from both the government and private sectors in Saudi Arabia fosters a more conducive environment for the uptake of innovative yet costly AMD treatments.
Market Restraints
High cost of treatments: The main treatment for wet AMD, anti-VEGF injections, is considerably costly. The cost of a vial of Lucentis solution is around US $1800 for cash-paying patients. This places a significant financial strain on the healthcare system and the patients. Additionally, insurance does not pay for these expenses, creating a financial barrier for patients.
Limited accessibility: Saudi Arabia faces a shortage of human resources, especially trained ophthalmologists, for accurate AMD diagnosis and prompt treatment. Newer therapies, such as gene therapies and stem cell therapy, are currently unavailable there, which makes access difficult.
Treatment limitations: Regular administration of anti-VEGF injections, whether monthly or bi-monthly, causes inconvenience for patients, leading to non-adherence and reduced treatment effectiveness. Additionally, some patients encounter unfavourable reactions to the medication, exacerbating their reluctance to follow the treatment regimen, thereby constraining market growth.
Healthcare Policies and Regulatory Landscape
Vision 2030 is propelling a substantial overhaul in Saudi Arabia's healthcare sector. Entities such as the Ministry of Health, the Council of Cooperative Health Insurance, and the SACB actively mold this landscape, already effecting significant changes, notably in enforcing mandatory health insurance for expatriates. These transformations offer extensive prospects for pharmaceutical firms. Anticipated alterations encompass heightened healthcare expenditure, infrastructure enhancements, and potential privatization, poised to further reconfigure the regulatory framework.
Securing licenses, particularly in pharmacies, now demands more stringent procedures as outlined by the Saudi Food and Drug Authority (SFDA). Keeping abreast of evolving regulations becomes pivotal for pharmaceutical businesses to leverage the developing Saudi healthcare surge.
Competitive Landscape
Key Players
- Regeneron Pharmaceuticals
- Novartis AG
- F. Hoffman-La-Roche Ltd
- Apellis Pharmaceuticals
- AbbVie
- Bayer
- Pfizer Inc
- Regenxbio Inc
- Kanghong Pharma
- Bausch Health Companies, Inc
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Saudi Arabia Age-Related Macular Degeneration Therapeutics Market Segmentation
By Disease Type
- Dry AMD
- Wet AMD
By Drug
- Lucentis
- Eylea
- Beovu
By Age Group
- Less than 60
- Between 60-80
- More than 80
By Stage
- Early AMD
- Intermediate AMD
- Advanced AMD
- No AMD
By Distribution Channel
- Hospital Pharmacy
- Specialty Pharmacy
- Online Pharmacy
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.