Saudi Arabia 3D Printing Medical Devices Market Analysis
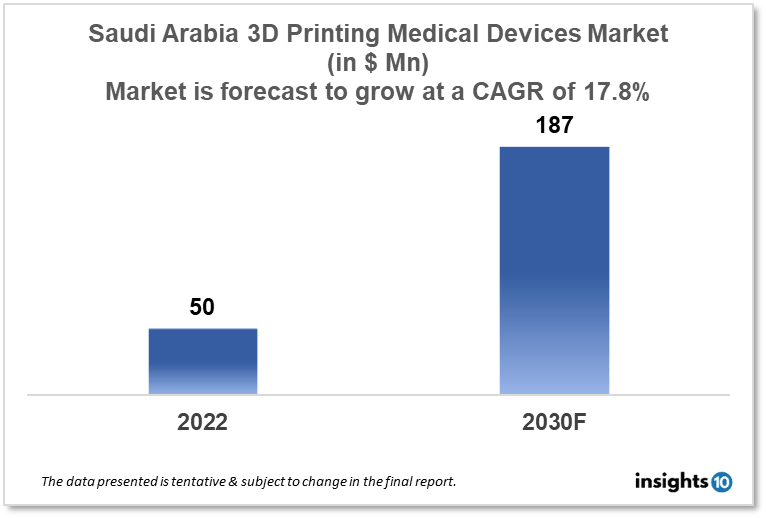
Saudi Arabia 3D Printing Medical Device Market is expected to witness growth from $50 Mn in 2022 to $187 Mn in 2030 with a CAGR of 17.80% for the forecasted year 2022-2030. In Saudi Arabia, the prevalence of chronic diseases like diabetes, cancer, and cardiovascular disease is rising. As a result, there is an expanding need for 3D-printed medical devices that can be used for the diagnosis and treatment of these diseases. The market is segmented by application, by technology and by end user. Some key players in this market include: Saudi Print 3D, Al Amin Medical Instruments, GE Healthcare, Stryker and Siemens Healthineers
Buy Now

Saudi Arabia 3D Printing Medical Devices Market Executive Summary
The Saudi Arabia 3D Printing Medical Devices Market size is at around $50 Mn in 2022 and is projected to reach $187 Mn in 2030, exhibiting a CAGR of 17.80% during the forecast period. Saudi Arabia's 2023 budget will see a 4.5% decrease in health expenditures compared to 2022. Currently, 5.5% of GDP is spent on health in 2023. Male life expectancy in Saudi Arabia is 75.33 years, while female life expectancy is 78.56 years. Saudi Arabia's out-of-pocket spending as a percentage of total health spending in 2020 was 16.5%.
Saudi Arabia's healthcare industry is increasingly utilising 3D printing technology. By enabling the production of personalised medical devices that can cater to patients' individual requirements, 3D printing technology has revolutionised the field of medicine. Due to its many advantages and uses, 3D printing technology is increasingly being used in Saudi Arabia's medical and healthcare sector. The development of personalised prosthetics that precisely fit the patient's body is now possible credits to 3D printing technology. The development of surgical instruments and guides using 3D printing technology enables surgeons to carry out intricate operations with greater accuracy and precision. Researchers can design and test prototypes of novel medical devices using 3D printing technology before they are produced in large quantities.
Market Dynamics
Market Growth Drivers Analysis
In Saudi Arabia, the prevalence of chronic diseases like diabetes, cancer, and cardiovascular disease is rising. As a result, there is an expanding need for 3D-printed medical devices that can be used for the diagnosis and treatment of these diseases. As 3D printing technology quickly develops, manufacturers will be able to produce more intricate, complicated devices that are highly specialised to patient requirements. There will probably be more demand for 3D printed medical devices as the advantages of the technology become better recognised among patients and healthcare professionals The Saudi government has also made sizable investments in the healthcare sector, which include building new hospitals and healthcare centres and implementing cutting-edge medical technology like 3D medical imaging. The benefits of 3D medical imaging, such as the ability to identify illnesses earlier and the potential for less invasive treatments, are becoming more and more well known to patients in Saudi Arabia. As a consequence, it is anticipated that demand for these devices will increase in the upcoming years.
Market Restraints
A significant market inhibitor may be Saudi Arabia's absence of a comprehensive regulatory framework for 3D printed medical devices. It might be challenging for manufacturers to get certifications and approvals for their devices, which could cause a delay in both output and sales. Despite the fact that 3D printing technology has become more popular in the medical sector recently, Saudi Arabia has yet to embrace many 3D-printed medical devices. For producers, this can be difficult because they might have a hard time finding a place for their devices.
Competitive Landscape
Key Players
- Saudi Print 3D (SA)
- Al Amin Medical Instruments (SA)
- Medtronic
- Boston Scientific
- GE Healthcare
- Stryker
- Siemens Healthineers
Notable Recent Deals
2021: A collaboration between Al-Rushaid Technologies, a Saudi technology company, and Stratasys, a world leader in 3D printing, has been revealed in order to launch a 3D printing lab for medical uses in Saudi Arabia. The facility will use 3D printing technology to create specialised implants and other medical devices.
Healthcare Policies and Regulatory Landscape
In Saudi Arabia, 3D Printing Medical devices regulation is the responsibility of the Saudi Food and Drug Authority (SFDA). Guidelines for the registration and approval of medical devices, including those made using 3D printing technology, have been created by the SFDA. Before being sold and used in Saudi Arabia, medical devices must adhere to the SFDA's quality and safety guidelines. A legal framework for the manufacture and uses of 3D printed medical devices has also been created by the SFDA. The guidelines address topics like 3D printed medical device design, manufacturing, and testing. Before producing and marketing 3D printed medical devices in Saudi Arabia, manufacturers must receive SFDA clearance. In addition to the SFDA, the Ministry of Health (MOH) is in charge of governing Saudi Arabia's healthcare laws. The MOH is trying to encourage Saudi Arabia to use 3D printed medical devices. A national programme that supports healthcare innovation and promotes the creation of new medical technologies has been created by the ministry.
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
3D Printing Medical Devices Market Segmentation
By Component (Revenue, USD Billion):
The 3D Printing Medical Devices market is divided into equipment, materials, and software & services depending on the component. In 2020, the market for 3D printed medical devices was dominated by software and services. During the forecast period, the cost-effectiveness, utility, uniformity, and accuracy provided by services for medical device 3D printing are anticipated to drive the segment's expansion.
- Equipment
- 3D Printers
- 3D Bioprinters
- Materials
- Plastics
- Thermoplastics
- Photopolymers
- Metals and Metal Alloys
- Biomaterials
- Ceramics
- Paper
- Wax
- Other Materials
- Services & Software
By Application (Revenue, USD Billion):
The market for 3D-printed medical devices is divided into wearable/implantable medical devices, other medical devices, standard prosthetics and implants, custom prosthetics and implants, tissue-engineered goods, surgical guides, and surgical tools based on the application. In 2020, the custom prosthetics and implants market sector held a greater market share. Biological materials (such skin and bones), plastics, ceramics, and metals are just a few of the materials that may be used to create highly customizable prosthetics and implants using 3D printing. The development of this market sector is anticipated to be fueled by 3D printing of custom implants, which is drawing in new investors and medical device businesses.
- Surgical Guides
- Dental Guides
- Craniomaxillofacial Guides
- Orthopedic Guides
- Spinal Guides
- Surgical Instruments
- Surgical Fasteners
- Scalpels
- Retractors
- Standard Prosthetics & Implants
- Orthopedic Implants
- Dental Prosthetics & Implants
- Craniomaxillofacial Implants
- Bone & Cartilage Scaffolds
- Ligament & Tendon Scaffolds
- Custom Prosthetics & Implants
- Tissue-engineered Products
- Hearing Aids
- Wearable Medical Devices
- Other Applications
By Technology (Revenue, USD Billion):
The market for 3D printing medical devices has been divided into various technological categories, including electron beam melting (EBM), laser beam melting (LBM), photopolymerization, droplet deposition or extrusion-based technologies, three-dimensional printing (3DP) or adhesion bonding, and others. The segment of these that accounted for the biggest market share in 2020 was laser beam melting (LBM). The significant market share of this sector is linked to the technology's expanding use in the dentistry sector and in the production of implants for minimally invasive surgery.
- Laser Beam Melting
- Direct Metal Laser Sintering
- Selective Laser Sintering
- Selective Laser Melting
- LaserCUSING
- Photopolymerization
- Digital Light Processing
- Stereolithography
- Two-photon Polymerization
- PolyJet 3D Printing
- Fused Deposition Modeling
- Multiphase Jet Solidification
- Low-temperature Deposition Manufacturing
- Microextrusion Bioprinting
- Droplet Deposition/Extrusion-based Technologies
- Electron Beam Melting
- Three-dimensional Printing/Adhesion Bonding/Binder Jetting
- Other Technologies
By End User (Revenue, USD Billion):
Hospitals and surgical centers, dentistry and orthopaedic clinics, academic institutions & research laboratories, pharma-biotech & medical device firms, and clinical research organizations make up the different end-user segments of the 3D printing medical devices market. The sector of hospitals and surgical centers held the biggest market share in 2020. The significant market share of this sector can be due to the increased uptake of cutting-edge medical technology by hospitals, the expansion of existing 3D printing facilities, and the rising affordability of 3D printing services.
- Hospitals & Surgical Centers
- Dental & Orthopedic Clinics
- Academic Institutions & Research Laboratories
- Pharma-Biotech & Medical Device Companies
- Clinical Research Organizations
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































