Romania Radiotherapy Market Analysis
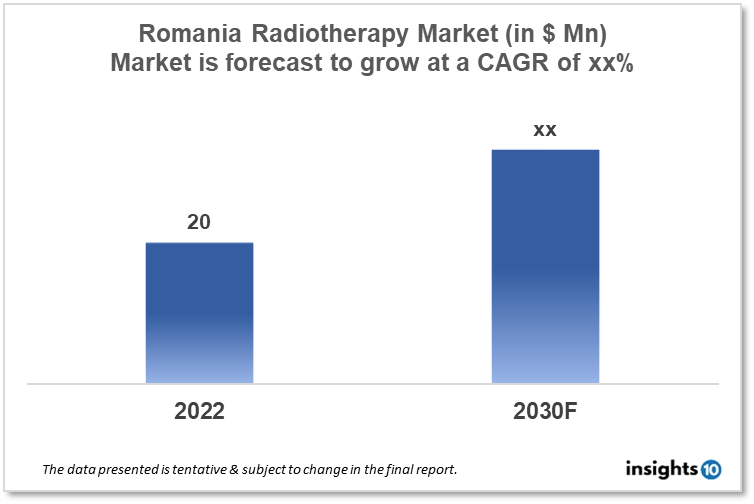
By 2030, it is anticipated that the Romania Radiotherapy Market will reach a value of $xx Mn from $20 Mn in 2022, growing at a CAGR of xx% during 2022-30. The Radiotherapy Therapeutics Market in Romania is dominated by a few domestic players such as Rotablator, Nuclearelectrica, and Radon Medical Services. The radiotherapy market in Romania is segmented into different types, technologies, procedures, applications, and end-users. The significant risk factors associated with awareness of radiotherapy shortage of skilled staff, government initiatives, and reimbursement policy. The demand for Romania Radiotherapy is increasing due to the rise in cancer cases in the country.
Buy Now

Romania Radiotherapy Market Analysis Summary
By 2030, it is anticipated that the Romania Radiotherapy Market will reach a value of $xx Mn from $20 Mn in 2022, growing at a CAGR of xx% during 2022-30.
Romania is an upper-middle-income, developed country located at the crossroads of Central and Southeastern Europe. In 2020, there will be 53,881 new cancer cases in males and 45,005 new cancer cases in women in Romania. The most common cancer types among men were lung cancer (16.8%) and other malignancies (41.5%). Breast cancer was the most prevalent (22.6% of females), followed by other cancers (41.7%).
Cancer mortality in Romania is 48% higher than in the rest of the EU, according to the Federation of Associations of Cancer Patients (FABC). The number of cancer cases in the European Union is expected to climb by 24% by 2035, making cancer the leading cause of death. Romania's government spent 6.3% of its GDP on healthcare in 2020.
Market Dynamics
Market Growth Drivers Analysis
Cancer is the second leading cause of mortality in Romania after cardiovascular disease and cancer treatments are sponsored under a special National Oncology Program. Romania has a broad and competitive industry due to its low labor costs. In 2001, the EU included Romania in its "Golden Growth" scheme. In Romania, there are about 100 radiation accelerators, with each accelerator treating 50-70 patients per day. Even though we have 100 accelerators, there are still waiting lists. The Oncology Institute "Of Prof. Dr Ion Chiricuta" Cluj-Napoca is one of Romania's top cancer treatment facilities. These aspects could boost Romania's, Radiotherapy Market.
Market restrains.
Cancer diagnosis, treatment, and follow-up expenses are covered by a national mandatory state insurance coverage in Romania. Individuals with cancer, however, continue to encounter several administrative and social obstacles as a result of coverage gaps. Despite major improvements in radiation infrastructure in Romania over the last 5-10 years, quality has been slow to improve, at least in the public sector. Romania has nearly increased the number of linear accelerators in the previous five years, closing the gap with Western Europe, although it still falls short of the European Society for Radiotherapy and Oncology's recommendation of one linear accelerator per 200,000 population. These factors may deter new entrants into the Romania Radiotherapy Market.
Competitive Landscape
Key Players
- Nuclearelectrica - a Romanian state-owned company that operates the Cernavoda nuclear power plant and produces medical isotopes used in radiation therapy
- Elekta Romania - a subsidiary of Elekta, a Swedish company that specializes in radiation therapy, radiosurgery, and brachytherapy equipment
- Varian Medical Systems Romania - a subsidiary of Varian Medical Systems, a US-based company that develops and manufactures equipment for cancer treatment, including radiation therapy
- Rotablator Romania - a Romanian company that provides radiation therapy services and also sells equipment and supplies
- Radon Medical Services - a Romanian company that provides radiation therapy services and also distributes equipment and supplies
Recent Notable Updates
April 2023: The Amethyst Centre in Cluj-Napoca now has the region's first MRI devoted to radiotherapy simulation, the MAGNETOM Sola RT Pro Edition from Siemens Healthineers, a leader in sophisticated medical technologies for over 125 years. This cutting-edge magnetic resonance imaging (MRI) system is specifically designed for oncology treatment and is based on advanced technologies and a high capacity to adapt to the needs of each patient.
Healthcare Policies and Reimbursement Scenarios
In Romania, radiotherapy is regulated by the National Commission for Nuclear Activities Control (CNCAN), which is responsible for ensuring the safe use of ionizing radiation in all areas, including medicine. Radiotherapy is partially reimbursed by the National Health Insurance Fund (CNAS), which is a government-funded health insurance scheme that provides coverage for a wide range of medical services, including radiotherapy.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Radiotherapy Segmentation
By Type (Revenue, USD Billion):
- External Beam Radiation Therapy
- Linear Accelerators
- Compact Advanced Radiotherapy Systems
- Cyberknife
- Gamma Knife
- ?Tomotherapy
- Proton Therapy
- Cyclotron
- ?Synchrotron
- Internal Beam Radiation Therapy
- Brachytherapy
- Seeds
- Applicators and Afterloaders
- Electronic Brachytherapy
- Systemic Radiation Therapy
- ?Others
?By Technology (Revenue, USD Billion):
- External Beam Radiotherapy
- Intensity-Modulated Radiation Therapy (IMRT)
- Image-Guided Radiation Therapy (IGRT)
- Stereotactic Radiation Therapy (SRT)
- 3D Conformal Radiation Therapy (3D-CRT)
- Particle Therapy
- Internal Beam Radiotherapy
- Brachytherapy
- High-Dose Rate Brachytherapy
- Low-Dose Rate Brachytherapy
- Image-Guided Brachytherapy
- Pulse-Dose Rate Brachytherapy
- Systemic Radiation Therapy
- Intravenous Radiotherapy
- Oral Radiotherapy?
By Application (Revenue, USD Billion):
- Breast Cancer
- Cervical Cancer
- Colon and rectum Cancers
- Stomach Cancer
- Lung Cancer
- Prostate Cancer
- Skin Cancer
- Liver Cancer
- Other types of cancer
By End User (Revenue, USD Billion):
- Hospitals
- Radiotherapy Centers & Ambulatory Surgery Centers
- ?Cancer Research Institutes
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.










































