Romania Cardiac Surgery Instruments Market Analysis
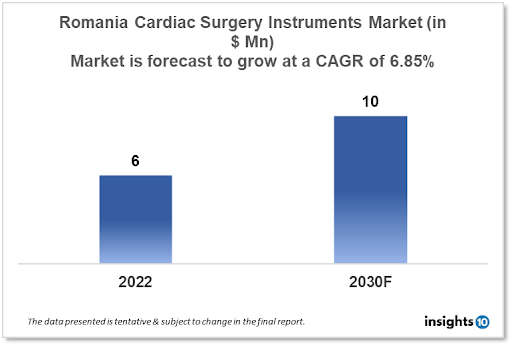
The Romania Cardiac Surgery Instruments Market is expected to witness growth from $6 Mn in 2022 to $10 Mn in 2030 with a CAGR of 6.85% for the forecasted year 2022-2030. Patients from different countries are travelling to Romania for cardiac surgeries, making it an increasingly popular location for medical tourism. The demand for high-quality cardiac surgery instruments is expected to increase as a result of this tendency. The market is segmented by type, application and by end user. Some key players in this market include BioSintex, Trident Medical Company S.R.L, LivaNova, B. Braun, Medline Industries, KLS Martin, and STILLE.
Buy Now

Romania Cardiac Surgery Instruments Healthcare Market Executive Analysis
The Romania Cardiac Surgery Instruments Market size is at around $6 Mn in 2022 and is projected to reach $10 Mn in 2030, exhibiting a CAGR of 6.85% during the forecast period. Romania's present health expenditures make up 6.3% of GDP in 2022. Romania, the EU member with the lowest expenditures, spent only $870 per individual on healthcare in 2015, or less than one-third of the EU average. The lowest GDP share linked to health is 6.3%, which is also the lowest, while the EU average is 9.9%, which is significantly higher.
In Romania, there are expected to be about 600,000 instances of cardiovascular disease (CVD) in 2021. Additionally, it was calculated that 455 deaths per 100,000 people were attributable to CVD, which is higher than the average death rate in the European Union. With 20% of the adult population in Romania suffering from CVD, the prevalence of the disease is also significant. With about 35% of people in Romania having high blood pressure, hypertension is a very common condition. Utilizing cardiac surgery instruments enhances surgical precision, reduces the likelihood of complications, and enhances patient results. Cardiac surgery tools are also used in minimally invasive treatments, which require fewer incisions, are more comfortable, leave fewer scars, and heal more quickly. Additionally, cardiac surgery tools are used to install pacemakers, which are tiny devices used to regulate the heart's rhythm and are inserted beneath the skin of the chest. Pacemakers are used to treat arrhythmias or abnormal heartbeats. Surgery is used to address the irregular heartbeat known as atrial fibrillation. Surgery is performed to restore a normal heart rhythm and reduce the chance of stroke. A surgical procedure known as Coronary Artery Bypass Grafting (CABG) uses instruments from cardiac surgery to circumvent coronary arteries that are obstructed or constrained. The procedure is used to improve blood flow to the heart and reduce the chance of heart attack.
Market Dynamics
Market Growth Drivers
Romania is becoming an increasingly well-liked destination for medical tourism as a result of the influx of patients from various nations seeking cardiac procedures there. As a consequence of this tendency, there will likely be a rise in the demand for high-quality cardiac surgery instruments. The development of cardiac surgery tools, such as minimally invasive surgery techniques, robotic-assisted surgeries, etc., is fueling the growth of the surgical tool market in Romania. These innovations are improving patient results, reducing hospital stays, and increasing surgical accuracy. Due to the high prevalence of cardiac diseases there, there is a high demand for tools used in cardiac surgery. The ageing population and unhealthy lifestyle choices are anticipated to cause the prevalence of cardiac diseases to keep increasing. The recent rise in healthcare spending in Romania has increased the demand for medical technology and tools, including instruments for cardiac surgery.
Market Restraints
The high cost of cardiac surgery instruments could be a major challenge for Romanian healthcare providers. Due to restricted funding for healthcare and low reimbursement rates, hospitals and clinics may not be able to buy costly devices. It can be difficult for new entrants to seize market dominance in Romania's market for cardiac surgery tools because there are several established competitors in the country.
Competitive Landscape
Key Players
- BioSintex (RO)
- Trident Medical Company S.R.L (RO)
- LivaNova
- B. Braun
- KLS Martin
- Medline Industries
- STILLE
Healthcare Policies and Regulatory Landscape
The healthcare system and the regulatory structure in Romania fall under the authority of the National Agency for Medicines and Medical Devices (NAMMD), which is responsible for examining the efficacy, quality, and safety of medical devices, including those used in cardiac surgery. The NAMMD is the country's oversight organization for medical devices, and it has oversight for implementing EU medical device policies and laws. The European Union's Medical Devices Regulation (MDR), which specifies standards for medical device efficacy, quality, and safety, acts as a foundation for Romania's regulatory policies pertaining to instruments used in cardiac surgery. Medical devices, including those used in cardiac surgery, are subject to a conformity evaluation process before being distributed in Romania in accordance with the MDR.
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Cardiac Surgery Instruments Market Segmentation
By Type (Revenue, USD Billion):
The market is divided into segments in this study based on the goods, applications, end users, and geographical areas. The market is divided into forceps, scissors, needle holders, clamps, and other cardiac surgery instruments based on the product. In 2017 the forceps category led the market, and it is anticipated that it will increase at the fastest rate going forward. The rise in heart surgeries and the frequent usage of forceps in most cardiac procedures are credited with the segment's strong growth.
- Forceps
- Vascular Forceps
- Grasping Forceps
- Other Forceps
- Needle Holders
- Scissors
- Clamps
- Other Cardiac Surgical Instruments
By Application (Revenue, USD Billion):
The market is further segmented by application into paediatric cardiac surgery, heart valve surgery, coronary artery bypass graft (CABG), and other applications. The Romania market's largest and fastest-growing application segment is CABG. This is mostly explained by the increased prevalence of heart illnesses and the consequent rise in surgical treatments. The second-largest category is heart valve surgery.
- Coronary Artery Bypass Graft (CABG)
- Heart Valve Surgery
- Pediatric Cardiac Surgery
- Other Applications
By End User (Revenue, USD Billion):
Based on the end user, the market is segmented into hospitals and cardiac centers, and ambulatory surgery centers. The hospitals and cardiac centers segment is expected to dominate the market for cardiac surgery instruments. Growth in this end-user segment can be attributed to the increasing incidence of cardiac and heart valve diseases and the subsequent increase in the number of cardiac surgery procedures.
- Hospitals and Cardiac Centers
- Ambulatory Surgery Centers
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































