Poland Oncology Therapeutics Market Analysis
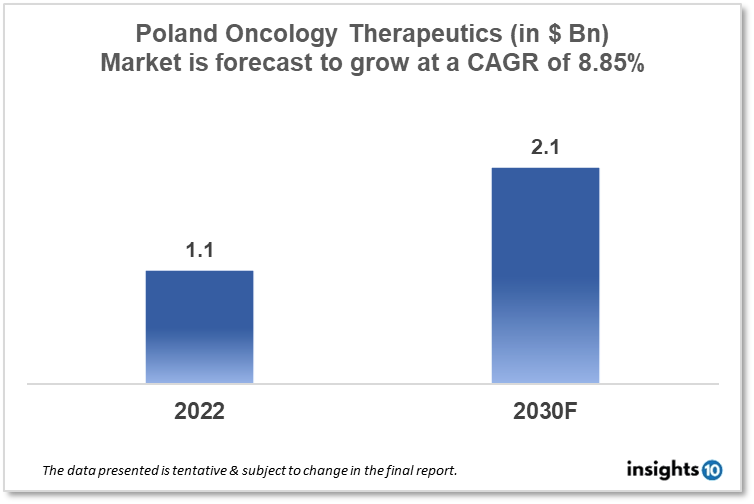
By 2030, it is anticipated that the Poland Oncology Therapeutics Market will reach a value of $2.1 Bn from $1.1 Bn in 2022, growing at a CAGR of 8.85% during 2022-30. The Oncology Therapeutics Market in Poland is dominated by a few domestic pharmaceutical companies such as Adamed Group, Polpharma Biologics, and OncoArendi Therapeutics. The Oncology Therapeutics Market in Poland is segmented into different types of cancer and different therapy types. The major risk factors associated with cancer are diet, alcohol, tobacco, air pollution, and physical inactivity. The demand for Poland Oncology Therapeutics is increasing on account of the rise in initiatives taken by the Government of the country.
Buy Now

Poland Oncology Therapeutics Market Analysis Summary
By 2030, it is anticipated that the Poland Oncology Therapeutics Market will reach a value of $2.1 Bn from $1.1 Bn in 2022, growing at a CAGR of 8.85% during 2022-30.
Poland is a high-income, developed country in Central Europe bordered by Belarus, the Czech Republic, Germany, Lithuania, Russia, Slovakia, and Ukraine. Cancer has a significant impact on public health in Poland, influencing healthcare expenses, burdening cancer survivors and their families, and continuing to be one of the major causes of mortality. In Poland in 2019, there were 171,218 incident cancer cases (85,559 males and 85,659 women) and 100,324 cancer deaths (54,370 among men and 45,954 among women).
Over the recent decade, cancer was the second biggest cause of death in Poland, accounting for over one-fourth of all male and female deaths. The Polish National Cancer Registry (PLCR) gathers, maintains, and administers information on all cancer cases diagnosed in the Polish population. The PLCR, which covers a population of 38.0 Mn people, provides vital information into cancer epidemiology in Central Europe. Poland's government spent 6.5% of its GDP on healthcare in 2020.
Market Dynamics
Market Growth Drivers Analysis
A rapid pathway for cancer patients was established in 2015 as part of a package of steps to shorten wait times for diagnostic tests and specialist appointments. The Polish government estimates that cancer treatment will cost $2.6 billion in 2020. These amounts included services given under the so-called oncology package, as well as parts of the services: clinical oncology, oncological surgery, oncological gynaecology and haematology, chemotherapy, radiation, and oncological medication programmes. In 2020, the Polish parliament passed the National Strategy for Oncology (NSO) 2020-2030, which aimed to promote evidence-based prevention, population screening programmes, appropriate treatment, and palliative care. These aspects could boost Poland's Oncology Therapeutics Market.
Market restraints
The conflict in Ukraine affects not just the country itself, but also countries where many Ukrainian refugees seek refuge, like Poland. Despite recent advances and advancements in cancer care in Poland, the system has limitations. The Polish National Cancer Registry (PNCR) is Poland's sole legally mandated population-based source of cancer incidence and prevalence data, recording both adult and children’s cancers. These factors may deter new entrants into the Poland Oncology Therapeutics Market.
Competitive Landscape
Key Players
- Adamed Group - Adamed Group is a Polish pharmaceutical company that specializes in the development and production of oncology drugs. They have a range of products for the treatment of various cancers, including breast cancer, lung cancer, and colorectal cancer
- Polpharma Biologics - Polpharma Biologics is a Polish biotechnology company that focuses on the development of biosimilars and novel biologics. They have a pipeline of oncology therapeutics, including biosimilars of rituximab, trastuzumab, and bevacizumab
- Celon Pharma - Celon Pharma is a Polish biopharmaceutical company that specializes in the development of drugs for oncology, neurology, and autoimmune diseases. They have a range of oncology products, including drugs for the treatment of breast cancer, lung cancer, and pancreatic cancer
- OncoArendi Therapeutics - OncoArendi Therapeutics is a Polish biopharmaceutical company that focuses on the discovery and development of small molecule drugs for the treatment of cancer and other diseases. They have a pipeline of oncology therapeutics, including drugs for the treatment of lung cancer and colorectal cancer
- Selvita - Selvita is a Polish biotechnology company that specializes in the discovery and development of drugs for oncology and other diseases. They have a range of oncology products, including drugs for the treatment of leukaemia and solid tumours
Recent Notable Deals
February 2023: The National Comprehensive Cancer Network (NCCN), a non-profit association of leading cancer centres in the United States, has announced a new collaboration with Poland's Institute of Haematology and Transfusion Medicine (IHIT) and the Alliance for Innovation—Polish-American Foundation (AFI). The three organisations have agreed to share their established knowledge and international experience in order to improve the quality of care and results for patients with hematologic malignancies in Poland and around the world.
Healthcare Policies and Reimbursement Scenarios
In Poland, the administrative body liable for supporting and observing oncology therapeutics is the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products (URPL). Concerningly, Poland has a public medical coverage scheme known as the National Health Fund (NFZ). The NFZ takes care of the expense of most oncology therapies for its selected individuals, including chemotherapy, radiation treatment, and designated treatment.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Oncology Therapeutics Segmentation
By Application (Revenue, USD Billion):
- Blood Cancer
- ?Colorectal Cancer
- Gastrointestinal Cancer
- Gynaecologic Cancer
- Breast Cancer
- Lung Cancer
- Prostate Cancer
- ?Others
By Drugs (Revenue, USD Billion):
- Revlimid
- Avastin
- Herceptin
- Rituxan
- Opdivo
- Gleevec
- Velcade
- Imbruvica
- Ibrance
- Zytiga
- Alimta
- Xtandi
- Tarceva
- Perjeta
- Temodar
- Others
By Therapy (Revenue, USD Billion):
- Chemotherapy
- Targeted Therapy
- Immunotherapy
- Hormonal Therapy
- Others
By Route of Administration (Revenue, USD Billion):
- Oral
- Parenteral
- Others
By Distribution Channel (Revenue, USD Billion):
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- ?Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.
By 2030, it is anticipated that the Poland Oncology Therapeutics market will reach a value of $2.1 Bn from $1.1 Bn in 2022, growing at a CAGR of 8.85% during 2022-2030.
In Poland, the administrative body liable for supporting and observing oncology therapeutics is the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products (URPL).
The Oncology Therapeutics Market in Poland is dominated by a few domestic pharmaceutical companies such as Adamed Group, Polpharma Biologics, and OncoArendi Therapeutics.








































