Poland ECG Equipments Market Analysis
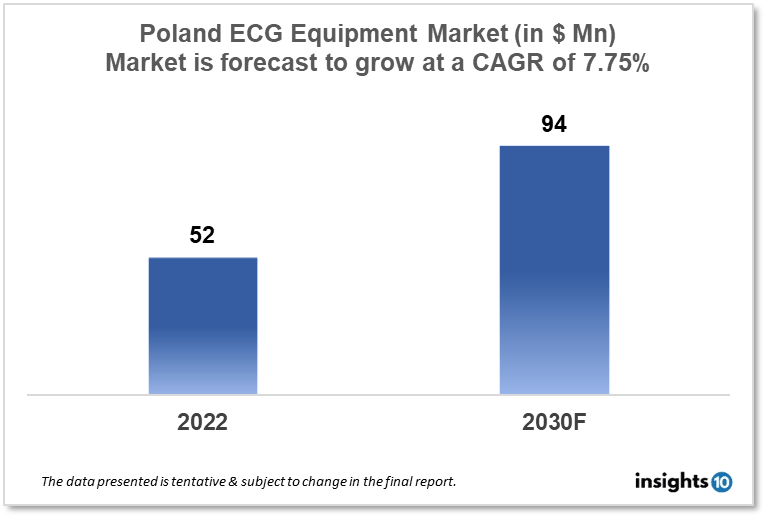
Poland's ECG Equipment Market is expected to grow from $52 Mn in 2022 to $94 Mn in 2030 with a CAGR of 7.75% for the forecasted year 2022-30. People in Poland are more concerned about their heart health as a result of increased knowledge and education about cardiovascular illnesses. Due to this, there is a higher need for routine checkups and preventative actions, raising the need for ECG equipment. The market is segmented by product type and by end user. Some key players in this market include Metrum Cryoflex, Skamex, Johnson & Johnson, Philipps Healthcare, Medtronic, GE Healthcare, and Nihon Kohden.
Buy Now

Poland ECG Equipment Healthcare Market Executive Analysis
Poland's ECG Equipment Market is expected to grow from $52 Mn in 2022 to $94 Mn in 2030 with a CAGR of 7.75% for the forecasted year 2022-30. In the EU, Poland has among the lowest health spending. Poland's 2020 health spending per person is $1347.23, or 6.3% of GDP, compared to the EU average of $3000, or 10.12% of GDP. Public funding only covers 72% of expenses, less than the EU average of 79%. The relatively expensive out-of-pocket expense (22%) raises accessibility concerns. Despite a drop from 23% in 2014 to 19% in 2023, Poland still has a greater prevalence of daily smokers among adults than most other European Union (EU) nations.
In Poland, almost 45% of fatalities in 2019 were caused by cardiovascular disease. An estimated 300,000 people in the nation suffer from heart failure, which is a major health concern. Arrhythmias, myocardial infarction, and heart failure are just a few of the disorders that can be diagnosed using ECG equipment. ECG measures the electrical activity of the heart and can reveal important details about the condition and operation of the heart. In patients with existing heart issues, ECG equipment is utilized to monitor the heart's health. By monitoring the electrical activity of the heart regularly, medical professionals can spot any changes or irregularities in the heart's function and modify treatment as necessary. A non-invasive ECG equipment testing technique does not call for any cuts or injections. As a result, patients of all ages can utilize it as a safe and simple test. In an emergency, having access to timely and precise findings from ECG equipment is essential. Doctors can promptly diagnose heart issues and offer prompt, efficient therapy.
Market Dynamics
Market Growth Drivers
People in Poland are more concerned about their heart health as a result of increased knowledge and education about cardiovascular illnesses. Due to this, there is now a higher need for routine checkups and preventative actions, which is raising the need for ECG equipment. Another significant element influencing the growth of the Polish market for ECG equipment is the country's aging population. The chance of developing cardiac illness rises as people become older, which has raised the demand for ECG equipment. Technology advancements are the main driver of the ECG equipment market in Poland. ECG devices are constantly being improved upon by manufacturers to become more precise, effective, and user-friendly. For instance, it is now simpler for clinicians to monitor patients and get their ECG findings remotely because of the development of wireless and portable ECG devices.
Market Restraints
Polish manufacturers may face competition from nations importing ECG equipment at lower prices. As a result, prices could be under pressure to drop, and domestic manufacturers might experience limited profitability. Many Polish healthcare workers probably lack access to finance for the acquisition of new equipment. Because it shrinks the market for their potential products, this presents a big problem for manufacturers.
Competitive Landscape
Key Players
- Metrum Cryoflex (PL)
- Skamex (PL)
- Johnson & Johnson
- Medtronic
- Philipps Healthcare
- GE Healthcare
- Nihon Kohden
Healthcare Policies and Regulatory Landscape
In Poland, the Ministry of Health is in charge of establishing healthcare laws and regulations. It is responsible for overseeing the Polish healthcare system and approving medical goods, such as ECG equipment, for use in Poland. The Ministry makes ensuring that medical equipment adheres to the necessary safety and quality requirements. Poland abides by the European Union's (EU) rules for medical devices as a member of the EU. The Medical Device Regulation (MDR), a collection of rules adopted by the EU in May 2021, is currently in force. This legislation lays out the guidelines for marketing medical products, such as ECG equipment, in the EU. It has tougher guidelines for clinical evidence, post-market monitoring, and medical device traceability. Before being sold in Poland, medical items, including ECG equipment, must bear the CE label. The CE certification certifies that the product conforms with EU medical device laws.
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
ECG Equipment Market Segmentation
By Product Type (Revenue, USD Billion):
- Holter Monitors
- Resting ECG Machines
- Stress ECG Machines
- Event Monitoring Systems
- ECG Management Systems
- Cardiopulmonary Stress Testing Systems
By End User (Revenue, USD Billion):
- Hospitals and Clinics
- Diagnostic Centres
- Ambulatory Services
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































