Poland Biomaterials in Healthcare Market Analysis
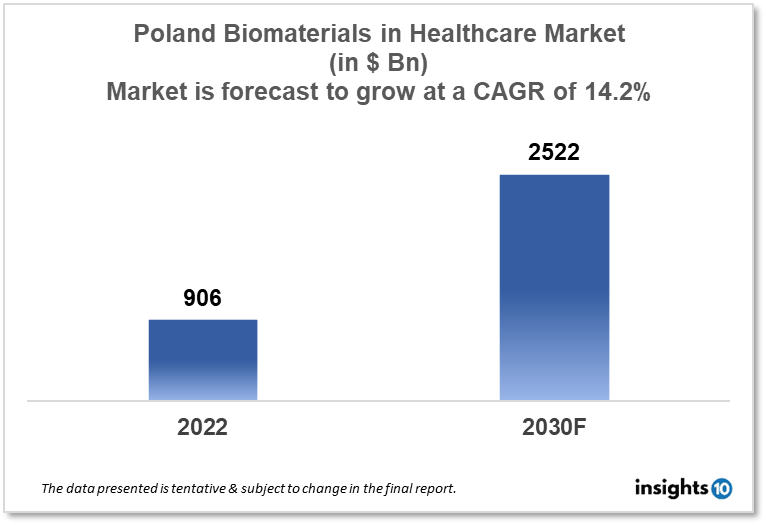
The Poland Biomaterials in Healthcare Market is expected to witness growth from $906 Mn in 2022 to $2522 Mn in 2030 with a CAGR of 13.65% for the forecasted year 2022-2030. Poland biomaterials manufacturers work with research organizations to create novel biomaterials or enhance existing ones. The market for biomaterials is expanding as a result of such collaboration that results in the creation of new products and invention. The market is segmented by type and by application. Some key players in this market include: NanoGroup S.A, Biovico, Matopat, BASF SE, Johnson and Johnson, Medtronic and Evonik Industries.
Buy Now

Poland Biomaterials in Healthcare Market Executive Analysis
The Poland Biomaterials in Healthcare Market size is at around $906 Mn in 2022 and is projected to reach $2522 Mn in 2030, exhibiting a CAGR of 13.65% during the forecast period. Poland has some of the lowest heath expenditures in the EU. (EU). Compared to the EU average of $3000, or 10.12% of GDP, Poland's 2020 health expenditure per individual is $1347.23, or 6.3% of GDP. 72% of costs are covered by public funds, which is less than the EU average of 79%. Accessibility concerns are raised by the comparatively high out-of-pocket expenditure (22%). Poland still has a higher incidence of daily smokers among adults than the majority of other European Union (EU) countries, despite a decline from 23% in 2014 to 19% in 2023.
In the Poland healthcare industry, biomaterials are used in a range of medical applications. When creating orthopedic devices like new knees and hips, biomaterials are frequently used. These implants have been created to closely resemble the characteristics of real bone, lowering the possibility of rejection and extending the life of the implant. Dental implants, which can restore lost teeth, are also created using biomaterials. These implants are made of biocompatible substances like titanium or zirconia that can integrate with the nearby bone to provide a secure foundation for the replacement tooth. Applications for biomaterials in wound healing include skin transplants and wound dressings. These substances support tissue regeneration and repair while acting as a barrier to shield against infection. Drugs can be delivered straight to a specific area of the body, such as a tumor, using biomaterials. The efficacy of the treatment can be increased while side effects are reduced thanks to this targeted drug delivery. The danger of rejection or allergic reactions is decreased because biomaterials are created to be biocompatible, which means they are not toxic or harmful to living tissue. Biomaterials are made to last for a very long time, minimizing the need for repeated repairs or operations. Biomaterials can be modified in terms of size, shape, or material to meet the unique requirements of a patient. By offering a solid foundation for tissue regeneration and reducing the chance of infection, biomaterials can facilitate the mending process.
Market Dynamics
Market Growth Drivers
Poland biomaterials manufacturers work with research organizations to create novel biomaterials or enhance existing ones. The market for biomaterials is expanding as a result of such collaboration that results in the creation of new products and invention. Poland's populace is getting older, and there are more senior citizens there who might need implants or other biomaterials. The demand for biomaterials is anticipated to rise as the population matures, propelling the biomaterials market's expansion. In order to improve access to treatment and modernize the healthcare system, the Polish government has recently increased healthcare spending. The demand for biomaterials is anticipated to rise as a result of this expenditure in healthcare. The market is anticipated to grow as a result of the development of new biomaterials and manufacturing techniques, which are expected to increase the quality and efficacy of biomaterials. In Poland, patients and healthcare workers are becoming more aware of biomaterials and their advantages. As a consequence, the market for biomaterials is anticipated to expand as demand for them rises.
Market Restraints
Poland's market for biomaterials is extremely cutthroat, with established players controlling the industry. For new entrants, gaining market share may be challenging due to this rivalry. Particularly for uncommon or specialized materials, the supply of raw materials for biomaterials may be constrained. This constrained supply may raise manufacturing costs and restrict the creation of biomaterials on a large scale. Patients and healthcare professionals may find biomaterials to be costly due to their high manufacturing costs. The adoption of biomaterials may be constrained by this cost factor, especially for patients with limited financial means.
Competitive Landscape
Key Players
- NanoGroup S.A (PL)
- Biovico (PL)
- Matopat (PL)
- BASF SE
- Johnson and Johnson
- Medtronic
- Evonik Industries
Recent Notable Deals
2022: A Poland medical packaging and sterilization business called Polymed was purchased by Pregis, a global provider of protective packaging solutions. The acquisition increased Pregis' global reach in the healthcare industry and opened doors for cooperation with biomaterials businesses.
2021: Two of Poland's ideal biomaterials businesses, Biovico and Matopat, merged to create the new business known as Biovico-Matopat. A stronger company with a wider variety of products and capabilities was produced as a result of the merger.
2021: A partnership contract was inked by NanoGroup and the Japanese biopharmaceutical firm PeptiDream. Using the peptide discovery technology of PeptiDream and the biomaterials knowledge of NanoGroup, the partnership intended to create novel biomaterials and medical devices.
Healthcare Policies and Regulatory Landscape
The regulatory authority in charge of regulating the registration and approval of biomaterials in Poland is the Polish Agency for Medical Devices (PAM). PAM evaluates register requests and makes sure that goods adhere to the rules outlined in the Act on Biomaterials. The rules for the manufacture, sale, and utilization of biomaterials are outlined in this legislation. Based on their intended use and degree of risk, medical devices are divided into four categories, and makers must adhere to the necessary regulations for each category. Biomaterials used in healthcare activities in Poland are reimbursed by the National Health Fund (NFZ). The Reimbursement Act, which explains the criteria for evaluating the medical and economic worth of products, regulates the reimbursement process. Based on the safety, efficacy, and cost-effectiveness of the biomaterials, the NFZ assesses their eligibility for compensation. In Poland, the State Agency for Health Technology Assessment (AOTMiT) assesses the efficacy and safety of biomaterials. Regarding the use of biomaterials in the healthcare system, including their status with regard to reimbursement, the agency makes suggestions to the Ministry of Health.
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Biomaterials in Healthcare Market Segmentation
By Type (Revenue, USD Billion):
Based on type, the market is segmented into Metallic Biomaterials, Polymeric Biomaterials, Ceramic Biomaterials, and Natural Biomaterials. The Metallic Biomaterials segment accounted for the largest share of the Poland market in 2019. The growing geriatric population Polandly is expected to drive growth for this segment.
- Metallic Biomaterials
- Stainless Steel
- Titanium & Titanium Alloys
- Cobalt-Chrome Alloys
- Gold
- Silver
- Magnesium
- Polymeric Biomaterials
- Polymethylmethacrylate
- Polyethylene
- Polyester
- Silicone Rubber
- Nylon
- Polyetheretherketone
- Other Polymeric Biomaterials
- Ceramics
- Calcium Phosphate
- Zirconia
- Aluminum Oxide
- Calcium Sulfate
- Carbon
- Glass
- Natural Biomaterials
- Hyaluronic Acid
- Collagen
- Gelatin
- Fibrin
- Cellulose
- Chitin
- Alginates
- Silk
By Application (Revenue, USD Billion):
The cardiovascular, orthopaedic, dental, plastic surgery, wound healing, tissue engineering, ophthalmology, neurological/CNS, and other applications segments are made up of the biomaterials market. The market category for wound healing is anticipated to have the highest CAGR in 2019. The market will increase as a result of factors including expanding healthcare infrastructure, a large population pool, a rising diabetic population, and rising healthcare spending. Surgical Guides
- Cardiovascular
- Catheters
- Stents
- Implantable Cardiac Defibrillators
- Pacemakers
- Sensors
- Heart Valves
- Vascular Grafts
- Guidewires
- Others
- Orthopedic
- Joint Replacement
- Knee Replacement
- Hip Replacement
- Shoulder Replacement
- Others
- Viscosupplementation
- Bioresorbable Tissue Fixation
- Spine?
- Spinal Fusion Surgeries
- Minimally Invasive Fusion Surgeries
- Motion Preservation & Dynamic Stabilization Surgeries
- Pedicle-Based Rod Systems
- Interspinous Spacers
- Artificial Discs
- Fracture Fixation Devices
- Bone Plates
- Screws
- Pins
- Rods
- Wires
- Synthetics Bone Grafts
- Joint Replacement
- Ophthalmology
- Contact Lenses
- Intraocular Lenses
- Functional Replacement of Ocular Tissues
- Synthetic Corneas
- Others
- Contact Lenses
- Dental
- Dental Implants
- Dental Bone Grafts & Substitutes
- Dental Membranes
- Tissue Regeneration
- Plastic Surgery
- Soft-Tissue Fillers
- Craniofacial Surgery
- Wound Healing
- Wound Closure Devices
- Sutures
- Staples
- Surgical Hemostats
- Internal Tissue Sealants
- Adhesion Barriers
- Hernia Meshes
- Wound Closure Devices
- Tissue Engineering
- Scaffolds for Regenerative Medicine
- Nanomaterials for Biosensing
- Tailoring of Inorganic Nanoparticles
?
- Neurological/Central Nervous System Applications
- Shunting Systems
- Cortical Neural Prosthetics
- Hydrogel Scaffolds for CNS Repair
- Neural Stem Cell Encapsulation
?
- Other Applications
- Drug Delivery Systems
- Gastrointestinal Applications
- Bariatric Surgery
- Urinary Applications
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.









































