Philippines Conjunctivitis Therapeutics Market Analysis
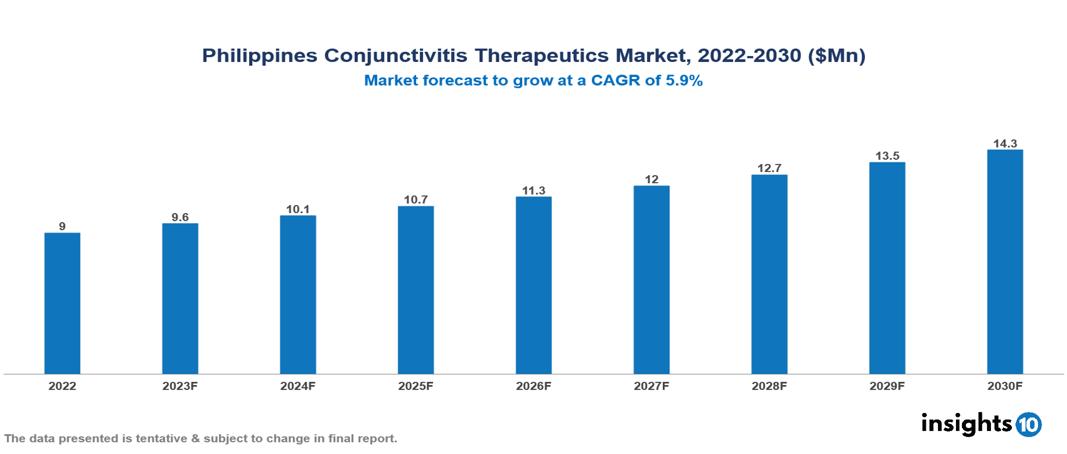
The Philippines Conjunctivitis Therapeutics Market was valued at $9 Mn in 2022 and is predicted to grow at a CAGR of 5.9% from 2023 to 2030, to $14 Mn by 2030. The key drivers of this industry include the rising prevalence of conjunctivitis, technological advancements in the industry, and rising disposable income. The industry is primarily dominated by players such as Allergan, Bausch & Lomb, Novartis, Rohto, Santen, and Johnson & Johnson among others.
Buy Now

Philippines Conjunctivitis Therapeutics Market Analysis Executive Summary
The Philippines Conjunctivitis Therapeutics Market is at around $9 Mn in 2022 and is projected to reach $14 Mn in 2030, exhibiting a CAGR of 5.9% during the forecast period.
Conjunctivitis, commonly referred to as pink eye, is the inflammation of the thin membrane covering the eyelids and the whites of the eyes. This condition can be triggered by various factors, including viruses, bacteria, allergies, and irritants. Viral conjunctivitis, often associated with the adenovirus responsible for the common cold, is highly contagious and typically initiates in one eye before spreading to the other. Symptoms vary based on the underlying cause but generally include redness in the whites of the eyes, a gritty sensation, excessive tearing, itching, swollen eyelids, and discharge. Although conjunctivitis often resolves on its own within a few weeks, it is crucial to seek medical attention if severe pain, changes in vision, light sensitivity, persistent discharge, or worsening symptoms occur. Treatment is tailored to the specific cause of conjunctivitis. Managing symptoms with pain relievers and cool compresses is common for viral cases, while bacterial cases may require antibiotic eye drops. Allergic conjunctivitis is typically addressed with antihistamine eye drops or oral medications. Companies such as Alcon, Allergan, Bausch & Lomb, Pfizer, and Santen Pharmaceutical are among those producing these treatments.
The Philippines faces an increased burden of infectious conjunctivitis which affects around 20% of the population. Market growth is propelled by significant factors such as the rise in the prevalence of conjunctivitis, technological advancements in the industry, and rising disposable income. However, conditions such as affordability challenges, lack of human resources, and dominance of generic medications restrict the growth and potential of the market.
Market Dynamics
Market Growth Drivers
Rising prevalence of conjunctivitis: Conjunctivitis is prevalent in the Philippines due to several factors, including the tropical climate where warm and humid conditions create an environment conducive to the growth of bacteria and viruses associated with conjunctivitis. Infectious conjunctivitis affects around 20% of the population. Additionally, exposure to air pollution, such as dust, smoke, and other pollutants, can irritate the eyes, contributing to the occurrence of conjunctivitis and increasing the demand for effective treatment.
Rising disposable income: The Philippine economy is steadily expanding, resulting in increased disposable incomes for individuals. This financial growth enables people to allocate more resources to healthcare expenses, including the purchase of medications for conditions like conjunctivitis. Moreover, government initiatives aimed at expanding healthcare access and enhancing insurance coverage are anticipated to contribute to the continued growth of the market.
Technological advancements: Pharmaceutical companies are consistently working on the development of enhanced treatments for conjunctivitis. These efforts involve the development of new drug formulations and advancements in delivery systems such as more effective and convenient eye drops and gels. Additionally, combination therapies are being explored, combining different medications to address various aspects of the infection. Furthermore, solutions for antimicrobial resistance are being sought to tackle the increasing concern of antibiotic resistance.
Market Restraints
Affordability challenges: The Philippines has a comparatively low per capita healthcare expenditure in contrast to developed countries, which limits the availability of advanced and costly conjunctivitis treatments. The expense of imported branded medications can pose a considerable challenge for patients, particularly in rural areas. This challenge is further intensified by the absence of universal health coverage and limited insurance penetration. Patients frequently shoulder a significant portion of healthcare costs, serving as a hindrance to obtaining essential treatments, particularly for persistent or recurring conditions like conjunctivitis.
Dominance of generic medications: Generic formulations, characterized by lower prices, tend to dominate the market, although they may lack significant brand recognition or perceived effectiveness compared to branded medications. The market for conjunctivitis therapeutics in the Philippines may face limitations in growth potential due to the relatively smaller population size compared to larger markets.
Workforce shortage: The shortage of well-trained ophthalmologists, especially in rural regions, may restrict the availability of specialized care for individuals with conjunctivitis. Inadequate training and awareness among healthcare professionals regarding the diagnosis and management of conjunctivitis can result in misdiagnosis and inappropriate treatment. High turnover rates among healthcare professionals can cause continuity problems and disrupt the provision of patient care.
Healthcare Policies and Regulatory Landscape
The main regulatory body overseeing drugs and pharmaceuticals in the Philippines is the Food and Drug Administration (FDA). The FDA is responsible for ensuring the safety, efficacy, and quality of food, drugs, and other health-related products in the country.
To obtain licensure for drugs and pharmaceuticals, manufacturers and distributors must undergo a rigorous evaluation process conducted by the FDA. This process involves the submission of comprehensive documentation, including details about the product's formulation, manufacturing processes, and clinical trial data. Once a product successfully passes the evaluation, the FDA issues the necessary licenses and permits for its distribution and sale in the Philippines.
For new entrants in the pharmaceutical industry, navigating the regulatory environment in the Philippines involves understanding and adhering to the stringent requirements set by the FDA. The regulatory process aims to protect public health by ensuring that only safe and effective pharmaceutical products enter the market.
Competitive Landscape
Key Players
- Allergan
- Alcon
- Bausch & Lomb
- Novartis
- Johnson & Johnson
- Zuellig Pharma
- United Laboratories
- Pascual Laboratories
- Rohto Pharmaceuticals
- Santen Pharmaceuticals
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Philippines Conjunctivitis Therapeutics Market Segmentation
By Drug Class
- Antibiotics
- Antiviral
- Antiallergic
- Others
By Treatment
- Mast Cell Stabilizers
- Decongestant
- Immunotherapy
- Antihistamines
- Non-steroidal anti-inflammatory drugs
- Olopatadine
- Epinastine
- Others
By Disease Type
- Bacterial
- Chemical
- Viral
- Allergic
By Formulation
- Ointment
- Drops
- Drugs
By End Users
- Hospitals and clinics
- Online Pharmacies
- Retail Pharmacies
- Drug Stores
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.