Philippines Brugada Syndrome Market Analysis
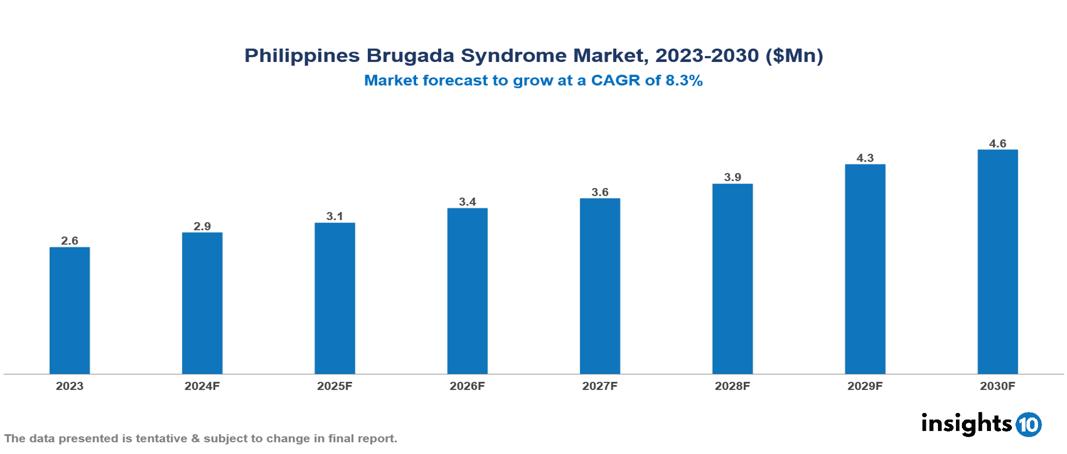
The Philippines Brugada Syndrome Market was valued at $2.6 Mn in 2023 and is predicted to grow at a CAGR of 8.3% from 2023 to 2030, to $4.6 Mn by 2030. The key drivers of the market include growing prevalence of cardiovascular diseases, geriatric population, and advancements in genetic testing. The prominent players of the Philippines Brugada Syndrome Market are Ajanta, Pascual Laboratories, Bauch Laboratories, Uni-Pharma Phils, Roche, and Medtronic, among others.
Buy Now

Philippines Brugada Syndrome Market Executive Summary
The Philippines Brugada Syndrome Market is at around $2.6 Mn in 2023 and is projected to reach $4.6 Mn in 2030, exhibiting a CAGR of 8.3% during the forecast period.
Brugada syndrome is a rare, but potentially threatening, genetic condition that causes abnormal electrical activity in the heart, leading to an increased risk of sudden cardiac death. People with Brugada syndrome have an increased risk of irregular heart rhythms beginning in the lower chambers of the heart, i.e., the ventricles. Common signs and symptoms associated with Brugada Syndrome include dizziness, fainting, gasping and laboured breathing, particularly at night, irregular heartbeats or palpitations, extremely fast and chaotic heartbeat, and seizures. The risk factors for Brugada syndrome include family history of Brugada syndrome, being male, race, and fever.
The Philippines Brugada Syndrome Market is driven by significant factors such as growing prevalence of cardiovascular diseases, geriatric population, and advancements in genetic testing. However, high cost of treatment, limited awareness, and limited R&D restrict the growth and potential of the market.
The major players of the Philippines Brugada Syndrome Market are Ajanta, Pascual Laboratories, Bauch Laboratories, Uni-Pharma Phils, Roche, and Medtronic, among others.
Market Dynamics
Market Growth Drivers
Growing Prevalence of Cardiovascular Diseases: According to the Philippine Statistics Office (PSA), CVDs are part of the larger group of noncommunicable diseases (NCDs), which accounted for 72% of deaths in the country in 2021. The rise in cardiovascular problems associated with age, lifestyle choices, and conditions like diabetes and hypertension has brought attention to uncommon hereditary diseases like Brugada syndrome. Investments in Brugada syndrome research, new diagnostic techniques, and therapies are being spurred by this increasing focus. The growing need for efficient treatments and management of cardiovascular diseases is propelling market development as healthcare systems place a higher priority on providing comprehensive cardiovascular care.
Geriatric Population: As of 2023, 6% of the total population in Philippines was accounted by the individuals aged 65 years and above. The prospective patient population grows as a result of an increased risk of cardiac diseases, particularly Brugada syndrome, in older adults. Furthermore, arrhythmia problems are more common in older persons, which emphasizes the necessity for efficient treatments and prevention measures. With more older people developing Brugada syndrome, there will be a greater need for patient monitoring devices, therapeutic approaches, and diagnostic instruments. This drives market expansion.
Advancements in Genetic Testing: Due to sophisticated technology, specific genetic abnormalities associated with arrhythmias are identified, resulting in earlier and more accurate diagnosis. In order to put preventative measures into place and lower the risk of sudden cardiac death, early identification is essential. Furthermore, genetic testing facilitates risk assessment for family members, encouraging early detection and treatment. The number of patients receiving diagnoses and the need for related therapies, equipment, and monitoring services are predicted to rise with the rising affordability and accessibility of genetic testing. This is anticipated to propel the expansion of the Brugada Syndrome Market.
Market Restraints
High Cost of Treatment: A key obstacle to the market's expansion is the high expense of Brugada syndrome therapy and diagnostics. Many patients are unable to afford advanced therapies, such as implanted cardioverter defibrillators (ICDs) and specialist genetic testing, which is especially true for individuals from lower- and middle-class families. The market's growth is slowed by this cost barrier, which limits access to necessary care and therapy, preventing early intervention, widespread diagnosis, and efficient management of Brugada syndrome.
Limited Awareness: In the Philippines, a significant barrier to the expansion of the market is the lack of knowledge about Brugada syndrome. The widespread lack of knowledge about the illness among patients, healthcare professionals, and the general public causes poor management, lost preventative chances, and delayed diagnosis. The lack of knowledge impedes the overall growth of the Brugada syndrome market in the nation by lowering the demand for diagnostic procedures, therapies, and patient support services.
Limited R&D: Limited research for Brugada syndrome significantly impedes market expansion. As a result of being a rare disease, the syndrome receives less funding than other cardiovascular disorders, which slows down the development of novel therapies, new diagnostic techniques, and a better knowledge of the illness. Because of this, advances in medicine are rare, which restricts the supply of efficient treatments and diagnostic techniques and, in turn, restrains the expansion of the Brugada Syndrome Market.
Regulatory Landscape and Reimbursement Scenario
The Food and Drug Administration (FDA) of the Philippines is a health regulatory agency under the Department of Health responsible for the licensing, monitoring, and regulation of the drugs, foods, cosmetics, vaccines, and medical devices in the Republic of the Philippines.
New drug products can enter the Philippines in one of two ways: either an Abridged Review or a Verification Review. Based on a previous evaluation from an RDRA (Reference Drug Regulatory Agency), an Abridged Review is a limited independent examination of particular sections of the dossier or submission for acceptability of use under local conditions and regulatory requirements. This can be used if the drug product, vaccine, or biological has already received approval from an RDRA and is within three years of that approval date. The Verification Review describes an evaluation process in which the FDA merely verifies the submission and confirms that the product complies with the standards, requirements, and registration conditions that have been approved by the RDRAs after the submission has been assessed and approved by at least two (2) RDRAs. The Abridged Review and Verification Review have an approval time period of not more 45 and 30 working days, respectively.
The reimbursement landscape for healthcare in the Philippines is complex and constantly evolving. The government of the Philippines provides funding for a public healthcare system. For some residents, especially the underprivileged, this system offers basic medical care at discounted costs or even without charge. Access to experts and cutting-edge treatments, however, may be restricted. Compared to the public sector, more services are offered by the private healthcare system, such as sophisticated procedures and diagnostics, reduced waiting periods, and choice of medical facilities and experts. A government-owned initiative, the Philippine Health Insurance Corporation (PhilHealth) provides employees in the formal sector and their dependents with a mandated national health insurance plan. A percentage of the cost of hospitalization and specific outpatient services in approved public and private hospitals is covered by PhilHealth. Next, the Social Amelioration Program (SAP) is a government initiative that offers low-income families financial support for a range of needs, including healthcare.
Competitive Landscape
Key Players
Here are some of the major key players in the Philippines Brugada Syndrome Market:
- Ajanta
- Pascual Laboratories
- Bauch Laboratories
- Uni-Pharma Phils
- Roche
- Medtronic
- Abbott Laboratories
- Johnson & Johnson
- Siemens Healthcare
- F. Hoffmann-La Roche
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Philippines Brugada Syndrome Market Segmentation
By Diagnosis
- Electrocardiogram
- Electrophysiology (Ep) Test
- Genetic Testing
By Treatment
- Implantable Cardioverter-Defibrillator
- Drug Therapy
By End User
- Hospitals
- Clinics
- Diagnostic Centres
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































