Philippines Bio-Implant Market Analysis
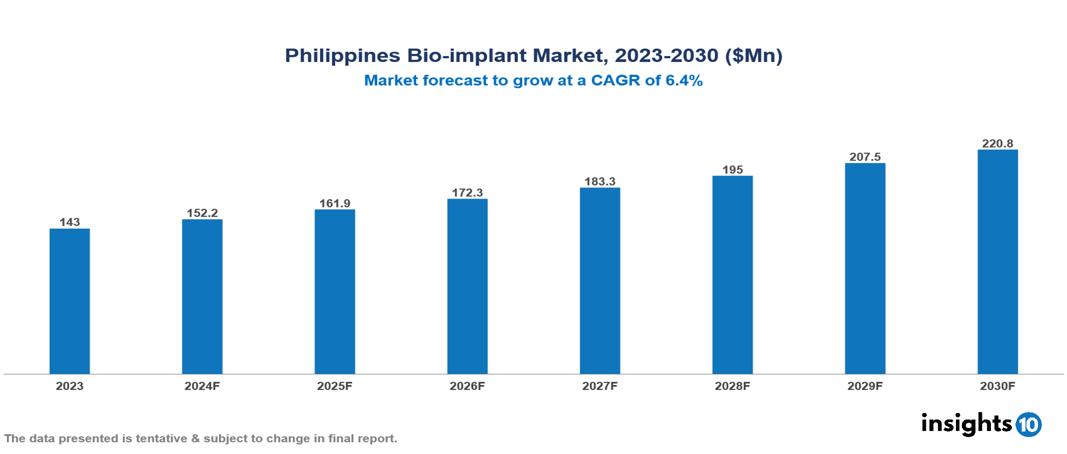
The Philippines Bio-implant Market was valued at $143 Mn in 2023 and is predicted to grow at a CAGR of 6.4% from 2023 to 2030, to 220.8 Mn by 2030. The Philippines Bio-implant Market is growing due to like the Aging Population, Growing Medical Tourism Industry, and Government Healthcare Initiatives. The market is primarily dominated by players such as Orthopaedic International, OrthoMed, Medline Philippines, Zimmer Biomet, Stryker Boston Scientific Corporation, Otto Bock Holding GmbH & Co. KG, and Medtronic plc.
Buy Now

Philippines Bio-implant Market Executive Summary
The Philippines Bio-implant Market is at around $143 Mn in 2023 and is projected to reach $220.8 Mn in 2030, exhibiting a CAGR of 6.4% during the forecast period.
Bioimplants, sophisticated medical devices designed for implantation in the body, aim to support or replace biological functions or structures. These devices range from basic dental implants to complex gadgets like pacemakers, prosthetic joints, and neurological implants. The main goal of bioimplants is to enhance the quality of life for individuals with different diseases or injuries by restoring lost or damaged functions. Constructed from biocompatible materials, bioimplants reduce the chance of rejection. The market is growing due to innovations such as wireless connectivity and sensors, which enable real-time monitoring and remote adjustments.
In the Philippines, the bioimplant market is influenced by a rising prevalence of chronic diseases, with 39.6% of adults having hypertension and 7.1% diagnosed with diabetes. The aging population is expanding, with 8.2% of the population aged 60 and above, expected to double by 2050. Demographic factors include a youthful median age of 25.7 years and a rapidly urbanizing society, with 47.4% living in urban areas. These factors drive demand for bioimplants but also present challenges in healthcare accessibility and affordability, impacting market growth. Therefore, the market is driven by significant factors like the Aging Population, Growing Medical Tourism Industry, and Government Healthcare Initiatives. However, Shortage of Skilled Professionals, Regulatory Hurdles, and Limited Insurance Coverage restrict the growth and potential of the market.
ZimVie launched the Food and Drug Administration clearing T3 pro tampered implant and Encode emergence Healing Abutment.
Market Dynamics
Market Growth Drivers
Aging Population: The Philippines has a rapidly aging population. As of 2023, approximately 7.5% of the population was aged 60 years and above, a figure expected to double by 2040. This demographic shift increases the demand for bioimplants, particularly for orthopedic and dental applications, as older adults are more prone to degenerative diseases and injuries requiring such implants.
Growing Medical Tourism: The Philippines is becoming a hub for medical tourism, attracting patients from neighboring countries due to the availability of high-quality medical care at relatively lower costs. The country hosted around 100,000 medical tourists, contributing significantly to the demand for advanced medical procedures and bioimplants.
Government Healthcare Initiatives: Government initiatives aimed at improving healthcare access and quality play a significant role in driving the bioimplant market. The Universal Health Care (UHC) Act, signed into law in 2019, aims to provide equitable access to quality health services for all Filipinos. The implementation of UHC is expected to increase the utilization of advanced medical treatments, including bioimplants, thereby boosting market demand.
Market Restraints
Shortage of Skilled Professionals: There is a notable shortage of trained healthcare professionals specializing in bioimplant procedures. This scarcity restricts the number of surgeries that can be performed, with only about 20% of hospitals in the country having the required expertise and facilities.
Regulatory Hurdles: The regulatory framework governing the approval and distribution of bioimplants in the Philippines can be cumbersome and time-consuming. Lengthy approval processes and stringent regulations can delay the introduction of new bioimplant products to the market. It is estimated that regulatory approvals can take up to 2-3 years, delaying patient access to the latest innovations.
Limited Insurance Coverage: Health insurance coverage in the Philippines is limited, and many insurance plans do not cover bioimplant procedures. This lack of coverage further exacerbates the affordability issue, as patients have to pay out-of-pocket. Only about 5% of the population has insurance plans that cover bioimplants, limiting the market's potential customer base.
Regulatory Landscape and Reimbursement Scenario
The regulatory landscape for the bioimplant market in the Philippines is governed by the Philippines Food and Drug Administration's (FDA) Center for Device Regulation, Radiation, Health, and Research (CDRRHR). The CDRRHR is responsible for the regulation of medical devices, including bioimplants, ensuring their safety, efficacy, and quality. This involves the evaluation of product registration applications, adherence to international standards, and compliance with Good Manufacturing Practices (GMP). Manufacturers and importers must obtain a Certificate of Product Registration (CPR) before marketing their products.
In the Philippines, the reimbursement scenario for bioimplants is evolving, with limited coverage under public health insurance programs such as PhilHealth. Most bioimplant procedures are financed out-of-pocket or through private health insurance, creating significant financial barriers for patients. Efforts to improve accessibility and affordability include expanding PhilHealth coverage and integrating bioimplant procedures into broader healthcare packages. However, the high costs and complex approval processes for reimbursement remain challenges for widespread adoption and equitable access to bioimplant treatments.
Competitive Landscape
Key Players
Here are some of the major key players in the Philippines Bio-implant Market:
- Orthopaedic International
- OrthoMed
- Medline Philippines.
- APEX Healthcare Berhad
- Hartalega Holdings Berhad
- Straumann AG
- Zimmer Biomet
- Stryker
- Boston Scientific Corporation
- Otto Bock Holding GmbH & Co. KG
- Medtronic
- Boston Scientific Corporation
- Johnson & Johnson Services, Inc.
- LifeNet Health
- Smith & Nephew
- Arthrex, Inc.
- Clinic Lemanic
- DePuy Synthes
- Exactech, Inc.
- Cochlear Ltd
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Philippines Bio-implant Market Segmentation
By Material
- Ceramics
- Polymers
- Alloys
- Biomaterials Metals
By Type
- Dental Bio-implants
- Orthopedic Bio-implants
- Spinal Bio-implants
- Ophthalmology Bio-implants
- Cardiovascular Bio-implants
- Others
By Mode of Administration
- Surgical
- Injectable
By End User
- Hospitals
- Speciality Clinics
- Ambulatory surgical centers
By Origin
- Autograft
- Allograft
- Xenograft
- Synthetic
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































