North America Breast Cancer Therapeutics Market Analysis
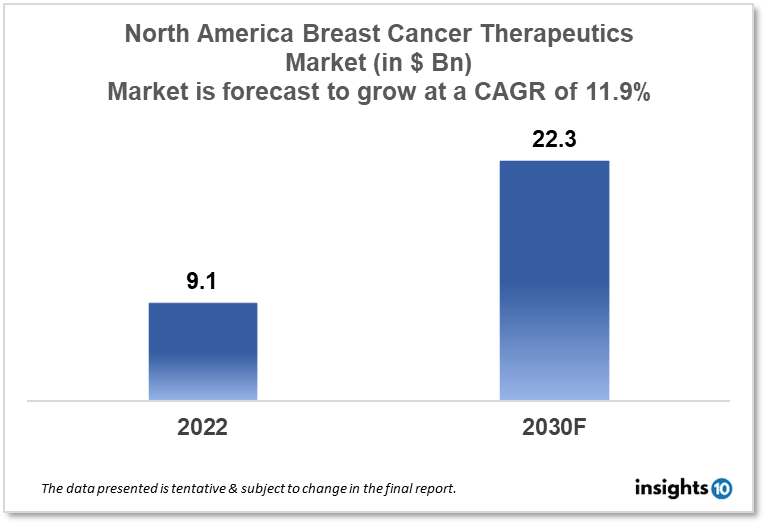
The North America breast cancer therapeutics market is expected to witness growth from $9.1 Bn in 2022 to $22.3 Bn in 2030 registering a CAGR of 11.9% for the year 2022-2030. The main market drivers for this growth are increasing awareness about breast cancer and post-menopausal obesity in women in North America. The market is segmented by therapy, by cancer type, and by distribution channel. AstraZeneca, Eisai, and Eli Lilly are some of the major market players in the North America breast cancer therapeutics market.
Buy Now

North America Breast Cancer Therapeutics Market Executive Analysis
The North America breast cancer therapeutics market size is at around $9.1 Bn in 2022 and is projected to reach $22.3 Bn in 2030, exhibiting a CAGR of 11.9% during the forecast period. The United States leads North America in terms of healthcare spending, accounting for a large %age of the region's GDP. It was predicted that 18% of the US GDP would be spent on healthcare in 2021. Both Canada and Mexico have high healthcare costs. Healthcare expenditures in North America aren't anticipated to rise nearly as quickly, despite average expected increases as high as 10% internationally next year due to inflation and higher healthcare consumption. The variations between 2022 and 2023 are a reflection of the wide range of North American trends that the major medical carriers have anticipated over the previous two years. Uncertain pandemic risks, shifting patterns of utilization, rising medical costs, and shifting prices throughout the healthcare system are all responsible for the variability.
In North America, a woman has a 13% chance of acquiring breast cancer at some point in her lifetime. According to this, a woman has a 1 in 8 probability of developing breast cancer. This also implies that she has a 7 out of 8 chance of never developing the illness. Since 1989, there has been a steady fall in the number of breast cancer deaths, with a projected 43% decrease by the year 2020. Better treatments as well as earlier detection of breast cancer through screening and more awareness are thought to be the causes of the decline in death rates. However, in recent years, the trend has marginally halted.
Enhertu (fam-trastuzumab-deruxtecan-nxki), an IV infusion, has been given approval by the U.S. Food and Drug Administration for the treatment of patients with metastatic or unresectable HER2-low breast cancer. This is the first medication that has been authorized specifically for patients with the HER2-low breast cancer subtype, a recently identified subtype of HER2-negative breast cancer. Enhertu is approved for HER2-low breast cancer patients who have already had chemotherapy for metastatic disease or whose cancer reappeared during or within six months after finishing adjuvant therapy. The DESTINY-Breast04 clinical trial, which included 557 adult patients with metastatic or unresectable HER2-low breast cancer, served as the foundation for this approval. It was a randomized, multicentre, open-label study. For this indication, Enhertu was given priority review and breakthrough treatment designations.
Market Dynamics
Market Growth Drivers
The North America breast cancer therapeutics market is expected to experience extraordinary growth in the coming years due to the increasing prevalence of breast cancer. This increase in demand will eventually force the North America breast cancer therapeutics market into a stable growth curve over the course of the projected period. In addition to this, the market will grow as more people adopt a western lifestyle, become more aware of breast cancer, quit nursing earlier, and experience an increase in postmenopausal obesity among women.
Market Restraints
The North America breast cancer therapeutics market may be impeded by issues like high treatment costs, a lack of qualified medical oncologists, and difficult and time-consuming therapeutic procedures.
Competitive Landscape
Key Players
- AstraZeneca
- Eisai
- Eli Lilly
- Roche
- Novartis
- Pfizer
Healthcare Policies and Regulatory Landscape
Although there are differences in coverage between nations and insurance plans, everyone in North America has access to comprehensive breast cancer health coverage. The Affordable Care Act mandates that private insurance plans in the US cover mammograms and other preventative care for women at no additional cost. But there are differences in coverage between states and insurance programs. In Canada, the national healthcare program (Medicare) pays for all required medical services, including the detection and treatment of breast cancer. Depending on the type of health insurance a person has, the extent of coverage for breast cancer treatment varies in Mexico. While private insurance plans might provide more in-depth coverage, public insurance plans might cover part or all of the expenses related to treating breast cancer.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Breast Cancer Therapeutics Segmentation
By Therapy (Revenue, USD Billion):
- Radiation Therapy
- Targeted Therapy
- Ribociclib
- Abemaciclib
- Afinitor
- Everolimus
- Trastuzumab
- Olaparib
- Ado-Trastuzumab Emtansine
- Palbociclib
- Pertuzumab
- Herceptin
- Tykerb (Lapatinib)
- Others
- Targeted Therapy
- Hormonal Therapy
- Selective Estrogen Receptor Modulators (SERMs)
- Aromatase Inhibitors
- Estrogen Receptor Downregulators (ERDs)
- Chemotherapy
- Taxanes
- Anthracyclines
- Anti-metabolites
- Alkylating Agents
- Immunotherapy
By Cancer Type (Revenue, USD Billion):
- Hormone Receptor
- HER+
By Distribution Channel (Revenue, USD Billion):
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.









































