Nigeria Oncology Clinical Trials Market Analysis
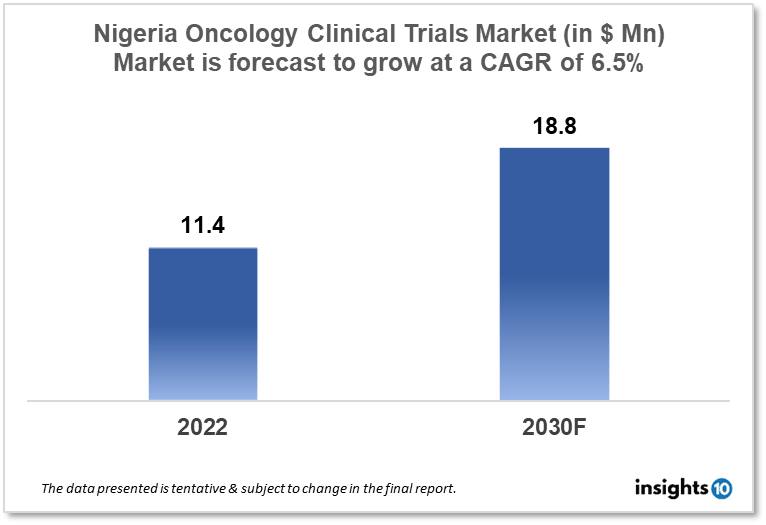
Nigeria's oncology clinical trials market is projected to grow from $11.4 Mn in 2022 to $18.8 Mn by 2030, registering a CAGR of 6.5% during the forecast period of 2022-30. The market will be driven by the high cancer burden, rising need for advanced cancer therapies, and rise in clinical trial financing. The market is segmented by phase, by study design & by indication. Some of the major players include Pfizer Inc., Roche Holding AG & Neimeth International Pharmaceuticals.
Buy Now

Nigeria Oncology Clinical Trials Market Executive Summary
Nigeria's oncology clinical trials market is projected to grow from $11.4 Mn in 2022 to $18.8 Mn by 2030, registering a CAGR of 6.5% during the forecast period of 2022-30. Nigeria's pharmaceutical sector imports a large portion of its processed raw materials. Moreover, large equipment and equipment utilized in the pharmaceutical business are made in other countries and imported into Nigeria. Because of the large population and low labor costs, the Nigerian pharmaceutical business is immense. Cancer kills around 72,000 people in Nigeria each year (30924 for males and 40647 for females). This figure is expected to rise since there are 102,000 additional cancer cases each year. Breast cancer (27%) is the most common, followed by cervix uteri (14%), liver (12%), prostate (12%), and colorectum (4.1%). Although breast cancer has a 20% death rate, the liver (16%), prostate (13%), cervix uteri (12%), and colorectum (4.4%). Breast cancer is currently the major cause of cancer mortality in Nigeria, followed by liver cancer and prostate cancer.
Breast, cervical, prostate, and colorectal cancer are the most frequent kinds of cancer in Nigeria. Unfortunately, the country's infrastructure and resources for oncology clinical trials are restricted, and the number of active studies is low in comparison to other parts of the globe. Notwithstanding these obstacles, certain oncology clinical trials are now underway in Nigeria, with the primary goal of increasing cancer screening and early diagnosis as well as researching novel treatment options There are few current phase II oncology studies in Africa, such as an investigator-initiated Phase II breast oncology clinical study in Nigeria that is assessing the response rate to subcutaneous trastuzumab. Although the oncology clinical trials landscape in Nigeria confronts various hurdles, rising cancer awareness and the desire for better treatment choices indicate that there is room for expansion in the sector. Nigeria might become a more active member in the global oncology clinical trials landscape with increasing investment in healthcare facilities and resources, as well as an emphasis on community participation and education.
Market Dynamics
Market Growth Drivers
Many reasons are driving the expansion of oncology clinical trials in Nigeria. To begin, Nigeria has a high cancer burden, with a large number of people in need of effective and affordable cancer therapies. This increases the desire for novel new treatments and therapies, which clinical trials may assist in developing. Clinical trials are becoming more popular with the Nigerian public, as well as among healthcare practitioners. As a result, patient enrollment for clinical trials has grown, as has cooperation between healthcare practitioners and research organizations. The Nigerian government has committed to upgrading healthcare infrastructure, including research and clinical trial capacity. This has resulted in a rise in clinical trial financing and resources, as well as the formation of collaborations between Nigerian research institutes and foreign organizations.
Market Restraints
There are various other constraints that may have an influence on the expansion of oncology clinical trials in Nigeria. The lack of infrastructure and resources, particularly access to contemporary diagnostic equipment and treatment facilities, is a serious barrier. This may make it challenging to perform clinical studies that fulfill international safety and effectiveness criteria. Another difficulty could be the scarcity of financing for clinical trials, which might restrict Nigerian researchers' capacity to perform high-quality studies. Moreover, there may be cultural or logistical challenges to participating in clinical trials, especially in rural communities with limited access to healthcare.
Competitive Landscape
Key Players
- Pfizer Inc.
- Roche Holding AG
- Novartis International AG
- AstraZeneca plc
- Merck & Co., Inc.
- Eli Lilly and Company
- Sanofi S.A.
- Bristol Myers Squibb Company
- GlaxoSmithKline (GSK)
- Neimeth International Pharmaceuticals (NGA)
Notable Insights
November 2022, in phase 1 clinical trials, an experimental breast cancer vaccine was shown to be safe. A novel plasmid Deoxyribonucleic Acid (DNA)/genetic material-based vaccination that may target a receptor in breast cancer is safe, according to recent phase 1 clinical trial findings.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Clinical Trials Regulation in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
6. Methodology and Scope
Oncology Clinical Trials Market Segmentation
By Phase (Revenue, USD Billion):
- Phase I
- Phase II
- Phase III
- Phase IV
By Study Design Outlook (Revenue, USD Billion):
- Epilepsy
- Parkinson's Disease (PD)
- Huntington's Disease
- Stroke
- Traumatic Brain Injury (TBI)
- Amyotrophic Lateral Sclerosis (ALS)
- Muscle regeneration
- Others
By Indication Outlook (Revenue, USD Billion):
- Interventional
- Observational
- Expanded Access
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































