Nigeria Coronary Stents Market Analysis
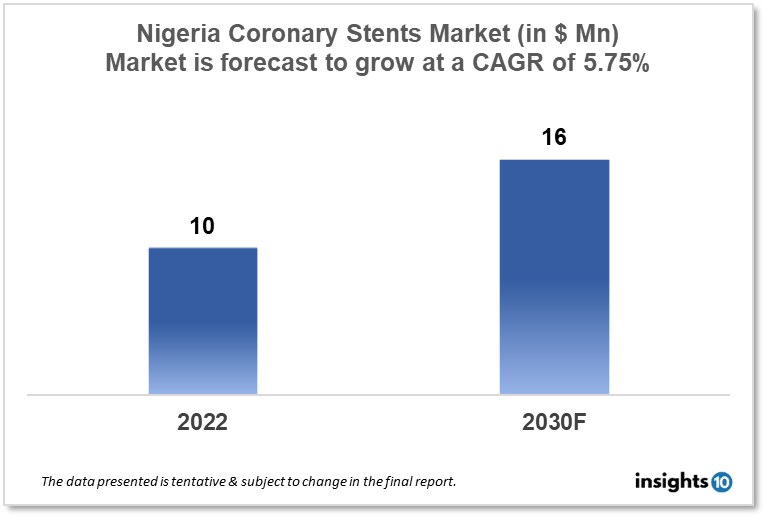
Nigeria's Coronary Stents Market is expected to witness growth from $10 Mn in 2022 to $16 Mn in 2030 with a CAGR of 5.75% for the forecasted year 2022-30. Minimally invasive procedures like Percutaneous Coronary Intervention (PCI) are becoming more popular in Nigeria due to their shorter hospital stays, quicker recoveries, and lower costs. This is driving the need for coronary stents as a crucial component of PCI operations. The market is segmented by type, mode of delivery, materials, and end user. Some key players in this market include AfricaMed, GBioMed, B. Braun, Abbott Laboratories, Biotronik, Medtronic, GE Healthcare, JNC International, Boston Scientific, Transmedia Healthcare, and Philips Healthcare.
Buy Now

Nigeria Coronary Stents Healthcare Market Executive Analysis
Nigeria's Coronary Stents Market is expected to witness growth from $10 Mn in 2022 to $16 Mn in 2030 with a CAGR of 5.75% for the forecasted year 2022-30. In Nigeria, the portion of the entire budget earmarked for health care is decreasing, going from 11.2% in 2020 to 8.6% in 2022. The amount spent on healthcare per person in Nigeria fell from $10.8 in 2020 to $8.5 in 2022. Between 2020 and 2022, Nigeria's health sector received a total share of capital that ranged between 49.8% and 53.9%.
In Nigeria, the cause of around 17862 deaths, or 12% of all deaths, was coronary heart disease. Over 570,256 people will be affected by CHD in Nigeria in 2022–2022, making it a country with a high prevalence of the disease. Older age groups had higher prevalence rates, with rates for men and women over 85 being the highest. In Nigeria, patients with coronary artery disease can use coronary stents, which are medical devices, to treat blocked or narrowed coronary arteries. By widening the restricted or obstructed coronary artery, coronary stents can restore blood flow to the heart muscle, which is their main advantage. This can lessen heart attack risk and relieve symptoms of chest pain. Occasionally, coronary stenting can eliminate the need for bypass surgery, a more invasive procedure with a protracted recovery period. When compared to bypass surgery, coronary stenting can be done as an outpatient procedure or with a shorter hospital stay, which lowers medical expenses. Coronary stenting can assist in reducing CAD symptoms including chest discomfort and breathlessness and enhance the quality of life for patients.
Market Dynamics
Market Growth Drivers
Minimally invasive procedures like Percutaneous Coronary Intervention (PCI) are becoming more popular in Nigeria due to their shorter hospital stays, quicker recoveries, and lower costs. This is driving the need for coronary stents as a crucial component of PCI operations. Technological advancements in coronary stent design and material composition are fueling consumer desire for newer and more advanced goods. Manufacturers who can offer innovative, high-quality goods are anticipated to gain market share in the Nigerian coronary stents market. CAD is growing more prevalent in Nigeria as a result of the country's ageing population and increased sickness prevalence. As a result, patients with CAD are increasingly choosing coronary stents as a form of therapy.
Market Restraints
Coronary stent manufacturers may be constrained by Nigeria's still-evolving healthcare infrastructure, which is particularly acute in rural areas. For a lot of patients in Nigeria, especially those without health insurance, the expense of CAD therapy, including PCI procedures and the usage of coronary stents, might be prohibitive. This may reduce the market's demand for medical equipment like coronary stents. Nigeria continues to be very reliant on foreign medical equipment, such as coronary stents. The market's accessibility and cost of medical equipment for patients and healthcare professionals may be constrained by the low local manufacturing capacity.
Competitive Landscape
Key Players
- AfricaMed (NG)
- JNC International (NG)
- GBioMed (NG)
- Biotronik
- Boston Scientific
- B. Braun
- Abbott Laboratories
- GE Healthcare
- Medtronic
- Philipps Healthcare
Healthcare Policies and Regulatory Landscape
The regulatory organisation in charge of the registration, oversight, and control of coronary stents in Nigeria is the National Agency for Food and Drug Administration and Control (NAFDAC). Before coronary stents may be sold in Nigeria, NAFDAC is in charge of making sure they adhere to a set of safety and efficacy requirements. The NAFDAC must receive any adverse events connected to coronary stent manufacturers' goods. This is crucial for monitoring the security and effectiveness of medical gadgets on the market. To import medical devices into Nigeria, manufacturers must adhere to import laws, which include getting the required licences and making sure the products fulfil the required safety and efficacy criteria.
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Coronary Stents Market Segmentation
By Type (Revenue, USD Billion):
The three main categories of coronary stents are bare-metal, drug-eluting, and bioabsorbable, depending on their nature. The segment for bioabsorbable stents is anticipated to increase at the fastest rate over the forecast period. The benefits that are encouraging the use of bioabsorbable stents include a decrease in problems including thrombosis and inflammation, the cNigeriaity to restore normal vasomotion, and an improvement in aberrant endothelial function. However, in the upcoming years, the expansion of this market segment is anticipated to be constrained by the high cost of bioabsorbable stents.
- Bare-metal Stents
- Drug-eluting Stents
- Bioabsorbable Stents
By Mode of Delivery (Revenue, USD Billion):
The market is divided into self-expanding and balloon-expandable stents according to delivery method. Because of the high use of these stents, rising research initiatives to advance the technology, and rising regulatory approvals for balloon-expandable stents, the balloon expandable stents segment is anticipated to increase at the greatest CAGR over the projection period.
- Balloon-expandable Stents
- Self-expanding Stents
By Materials (Revenue, USD Billion):
The coronary stent market is divided into metallic (cobalt chromium, platinum chromium, nickel-titanium, and stainless steel) and other stents according to the material. The market for other stents is anticipated to experience the largest CAGR growth during the projection period. The rapid expansion of this market can be ascribed to the increased use of polymers and copolymers in the production of bioabsorbable stents.
- Metallic Stents
- Cobalt-Chromium
- Platinum Chromium
- Nickel Titanium
- Stainless Steel
- Other Stents
By End User (Revenue, USD Billion):
The coronary stent market is divided into hospitals, cardiac centres, and ambulatory surgical centres based on the end user. According to predictions, the hospital sector will control the market in 2016. This is primarily caused by hospitals using a lot of coronary stents.
- Hospitals
- Cardiac Centers
- Ambulatory Surgical Centers
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.









































