Netherlands Oncology Drugs Market Analysis
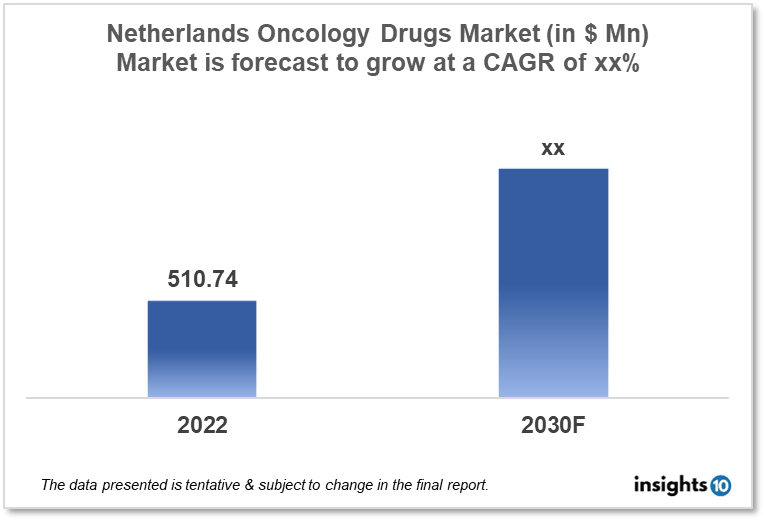
By 2030, it is anticipated that the Netherlands oncology drugs market will reach a value of $xx Mn from $510.74 Mn in 2022, growing at a CAGR of xx% during 2022-30. The oncology drugs market in the Netherlands is dominated by a few domestic pharmaceutical companies such as Cristal Therapeutics, ABL Europe, and Synthon. The oncology market in the Netherlands is segmented into different therapeutic areas and different treatment types. The major factors affecting the Netherlands oncology drugs market are the increasing disease burden of non-communicable diseases like cancer and the lack of healthcare facilities for cancer treatment in most of the areas of the Netherlands.
Buy Now

Netherlands Oncology Drugs Market Analysis Summary
By 2030, it is anticipated that the Netherlands oncology drugs market will reach a value of $xx Mn from $510.74 Mn in 2022, growing at a CAGR of xx% during 2022-30.
The Netherlands is a constituent high-income, developed country located in Western Europe and with territories in the Caribbean. Apart from Ireland and Denmark, the Netherlands has the highest number of cancer cases in Europe, with colon, melanoma, and breast cancer being the most frequent types of the disease. The Netherlands, like many other industrialised countries, has an ageing population, which is contributing to an increase in cancer incidence. The most prevalent types of cancer in the Netherlands are lung, breast, prostate, and colorectal cancer.
The Netherlands was one of the first countries to establish a dedicated cancer centre, the Netherlands Cancer Institute (NKI), which is currently regarded as one of the greatest comprehensive cancer clinics in the world. The Dutch government is investing in initiatives to improve cancer care in the Netherlands, such as funding for cancer research and the establishment of cancer specialist centres. These initiatives aim to improve cancer prevention, diagnosis, treatment, and support for patients. Netherlands' government spends 11.1 % of its GDP on healthcare.
Market Dynamics
Market Growth Drivers Analysis
For foreign investors, the establishment of home-grown multinational enterprises in the Netherlands, working with a dense network of SMEs, is particularly appealing. High-quality infrastructure and living levels increase purchasing power for cancer medications on the market. Women in the Netherlands are more likely to develop cancer, notably breast cancer and lung cancer, while men are more likely to develop prostate cancer than males in other European countries. These aspects could boost the Netherlands' oncology drugs market.
Market Restraints
In the Netherlands, payers such as health insurance companies can have a substantial impact on whether pharmaceuticals are covered and reimbursed. This may have an impact on the demand for oncology drugs as well as pharmaceutical companies capacity to generate money. In comparison to other nations, the Netherlands has a relatively small population, which may limit the market size for oncology treatments. These factors may deter new entrants into the Netherlands' oncology drugs market.
Competitive Landscape
Key Players
- Synthon (NLD)
- ABL Europe (NLD)
- Cristal Therapeutics (NLD)
- Merus (NLD)
- Kiadis Pharma (NLD)
- Hoffmann-La Roche Ltd.
- Novartis AG
- Gilead Sciences Inc.
- Bayer AG
Recent Notable Updates
January 2023: Lonza, a global manufacturing partner to the pharmaceutical, biotechnology, and nutrition industries, announced two new agreements with McSAF and Cristal Therapeutics to enhance its bioconjugates offering. The cooperation will allow two new conjugation platforms, McSAF Inside and CliCr, to be integrated into the Lonza Bioconjugation Toolbox. The Lonza Bioconjugation Toolbox includes a variety of specialised solutions for bioconjugate technology selection, development, and manufacture in order to accelerate the development of novel bioconjugate-based therapeutics.
May 2022: ABL Europe (ABL), a pure play contract development and manufacturing organisation (CDMO) specialising in virus development and manufacturing for vaccine candidates, gene and cancer therapies, and Odimma Therapeutics (Odimma), a young biotechnological company focusing on personalised cancer immunotherapy, have announced the signing of a development agreement. Odimma's revolutionary and patented vaccination platform ODI-2001 will be manufactured by ABL.
Healthcare Policies and Reimbursement Scenarios
The Dutch Medicines Evaluation Board (MEB) regulates oncology medications in the Netherlands. Before medications may be promoted or sold in the Netherlands, the MEB must assess their quality, safety, and efficacy. The MEB also regulates the import and export of oncology medications. Importers must have the appropriate licences and permissions, and pharmaceuticals shipped must meet the regulatory criteria of the recipient country.
Oncology medications and treatments are covered by the mandated basic health insurance offered by private health insurance firms in the Netherlands. Private insurers compete to provide coverage to Dutch residents under the Dutch healthcare system, which is based on a managed competition model. All medically necessary cancer medications and treatments included on the Dutch Healthcare Authority's List of Covered Drugs and Treatments must be covered by insurers. Furthermore, the Dutch government has established the Health Insurance Act Implementation Fund (Regeling Zorgverzekering) to give financial assistance for certain high-cost oncology drugs that are not covered in the List of Covered Drugs and Treatments.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Oncology Drugs Market Segmentation
By Drug class
- Cytotoxic drugs
- Alkylating agents
- Antimetabolites
- Others
- Targeted drugs
- Monoclonal antibodies
- Others
- Hormonal drugs
- Others
By Therapy
- Chemotherapy
- Targeted therapy
- Immunotherapy
By Indication
- Lung cancer
- Stomach cancer
- Colorectal cancer
- Breast cancer
- Prostate cancer
- Others
By Dosage form
- Solid
- Tablets
- Capsules
- Liquid
- Injectable
- Prefilled syringes
- Others
By Distribution channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.






































