Netherlands Cardiovascular Drugs Market Analysis
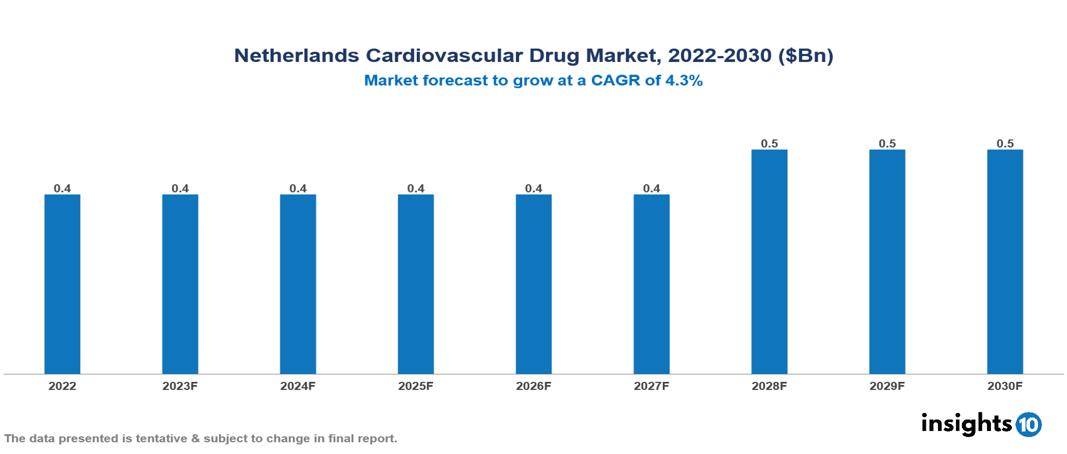
Netherlands Cardiovascular Drug Market is at around $0.4 Bn in 2022 and is projected to reach $0.5 Bn in 2030, exhibiting a CAGR of 4.3% during the forecast period. Increased elderly population, healthcare accessibility, and healthcare spending & affordability in the country are driving the growth of the market. The market is dominated by key players like Pfizer Inc., Novartis, Sanofi, Merck & Co., Astra Zeneca, Daiichi Sankyo, Takeda Pharmaceutical, Bristol-Myers Squibb Company, F. Hoffmann-La Roche Ltd, and Bayer AG.
Buy Now

Netherlands Cardiovascular Drug Market Executive Summary
Netherlands Cardiovascular Drug Market is at around $0.4 Bn in 2022 and is projected to reach $0.5 Bn in 2030, exhibiting a CAGR of 4.3% during the forecast period.
Netherlands Cardiovascular Drug Market Analysis is a thorough investigation of the country's cardiovascular pharmaceutical market. This study explores how the cardiovascular medicine industry is shaped by market trends, competitive dynamics, and regulatory factors. For those involved in the pharmaceutical and healthcare industries, the report offers critical insights by assessing variables such as medication development, market share, and therapeutic developments.
The Netherlands cardiovascular drug industry is distinguished by an emphasis on preventive cardiovascular care and an increasing need for novel medicines. Government measures strive to promote access to improved therapies, while major players invest in research and development to meet the rising prevalence of cardiovascular illnesses. Pharmaceutical businesses and healthcare institutions collaborate to improve patient outcomes, which shapes the competitive landscape of the market.
Development, manufacturing, and distribution of medicines are the focus of the pharmacy field. Global revenue for the pharmaceutical sector reached $138.33 Bn in 2022, a significant increase over the previous 20 years. Netherlands's domestic pharmaceutical market is growing quickly, which has led to many businesses creating and producing generic cardiovascular medications that are both highly effective and reasonably priced. The market becomes more competitive as a result.
Top companies in the market are Pfizer Inc., Novartis, Sanofi, Merck & Co., and Astra Zeneca. Pfizer is making significant investments in the study and creation of novel cardiovascular drugs, with a particular emphasis on personalized medicine and gene therapy for heart failure. The company is expected to retain its strong position in the Netherlands' cardiovascular medicine landscape due to its established presence in the market and its focus on innovation.
Market Dynamics
Market Growth Drivers:
Increased Elderly Population: The Netherlands' population is aging and the demand for cardiovascular medications may rise in response because cardiovascular diseases are more common in this age group. In the Netherlands 3.6 Mn people were 65 years of age or older in 2023. This amounts to 20.2% of the total populace.
Healthcare Accessibility: Early detection and treatment of cardiovascular disorders can be facilitated by an efficient and easily accessible healthcare system, which will increase demand for cardiovascular pharmaceuticals.
Healthcare Spending and Affordability: The population's capacity to pay for healthcare and prescription drugs, as well as economic stability, have an impact on market expansion. Spending enough on healthcare may increase the market for cardiovascular medications.
Market Restraints:
Reimbursement Challenges: Cardiovascular medication accessibility and pricing may be impacted by reimbursement issues. Drug prices and market penetration may be impacted by modifications to reimbursement policies.
Clinical Trial Failures: Potential growth of the market may be impacted by delays or cancellations of product launches due to setbacks in the clinical trials of new cardiovascular medications.
Global Health Events: Unpredicted occurrences like pandemics or health crises can wreak havoc on manufacturing, interrupt supply chains, and raise doubts about the market for cardiovascular drugs.
Healthcare Policies and Regulatory Landscape
Pharmacovigilance in the Netherlands is handled by the Medicines Evaluation Board Agency (MEB), which is also in charge of drafting and carrying out board decisions. It is within the control of the Welfare, Sport, and Health Ministry (VWS). Companies need to submit a pharmaceutical dossier to receive the marketing authorization. The safety and effectiveness of the medication are then evaluated. The process determines which organization evaluates this as the marketing authorization is issued by the Dutch Medicines Evaluation Board (CBG) for the Dutch market (national procedure). The study of how individual genetic differences affect drug response or pharmacogenomics has made the Netherlands a leader in this field. Companies may need to take into account how their drug interacts with various genetic profiles, which might complicate the approval process.
Competitive Landscape
Key Players:
- Pfizer Inc.
- Novartis
- Sanofi
- Merck & Co.
- Astra Zeneca
- Daiichi Sankyo
- Takeda Pharmaceutical
- Bristol-Myers Squibb Company
- F. Hoffmann-La Roche Ltd
- Bayer AG
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Netherlands Cardiovascular Drug Market Segmentation
By Drug Type
- Cephalosporins
- Antihypertensive
- Antihyperlipidemic
- Anticoagulants
- Antiplatelet Drug
- Others
By Disease Indication
- Hypertension
- Hyperlipidemia
- Coronary Artery Disease
- Arrhythmia
- Others
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































