Netherlands Breast Cancer Therapeutics Market Analysis
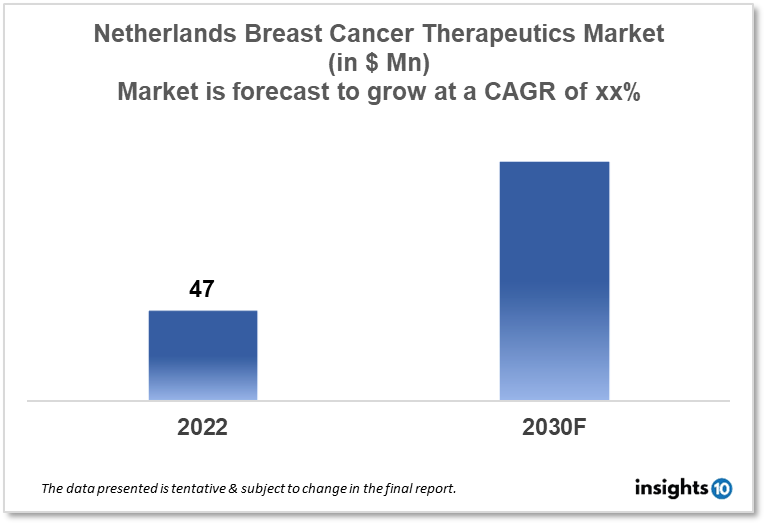
The Netherlands breast cancer therapeutics market is expected to witness growth from $47 Mn in 2022 to $xx Mn in 2030 with a CAGR of xx% for the year 2022-2030 due to market growth drivers like increasing awareness about the breast cancer therapies and rising research and development activities in the Netherlands. The market is segmented by therapy, by cancer type, and by distribution channel. Rousselot, Oncogenex, and Apthera are some of the major players in the Netherlands breast cancer therapeutics market.
Buy Now

Netherlands Breast Cancer Therapeutics Market Executive Analysis
The Netherlands breast cancer therapeutics market size is at around $47 Mn in 2022 and is projected to reach $xx Mn in 2030, exhibiting a CAGR of xx% during the forecast period. In 2022, the Netherlands spent $108.39 Bn on healthcare. The fight against the coronavirus received $2.28 Bn in funding the following year, which will cover vaccines, testing, and source and contact studies. In the coming year, $23.85 Mn was made available to help with pandemic preparedness. A national care reserve is to be established each year with a budget of approximately $5.42 Mn. In order to encourage firms to provide additional medical traineeships and internships, $68.83 Mn was made available in 2019. The Cabinet anticipates a $2.98 monthly premium increase for basic insurance. This will increase the monthly cost to almost $131.42. For those with lower earnings, who would see an increase in the health care allowance of $3.25 per month, the increase was neutralized. The $417.30 bare minimum deductible was not changed. A funding contribution of $5.96 Mn will support the 113-suicide prevention hotlines
Around 3,000 women pass away from breast cancer each year. In the Netherlands, 1 in 8 women will get breast cancer at some point in their lives. With a ten-year prevalence of 128,000, breast cancer is the most common type of cancer in the Netherlands. The course of treatment for breast cancer is determined by the stage, kind, and prognosis of the tumour. The average survival rate for women with breast cancer is over 87% for the first five years and over 77% for the next ten. Because of improved imaging and detection methods, screening programs, and the advent of novel medicines, survival rates have increased in Netherlands.
Most women with early-stage breast cancer in the Netherlands are candidates for mastectomy or breast-conserving surgery combined with radiation. With these methods, there is no distinction between the risk of local recurrence and the likelihood of survival. Axillary staging is done via sentinel node biopsy, and in women who test positive for sentinel nodes, axillary dissection is becoming less necessary. Molecular profiling to individualize treatment based on risk is now a clinical reality for patients with hormone receptor-positive malignancies. Adjuvant systemic therapy is used in the majority of women due to its shown benefit in terms of survival in Netherlands.
Market Dynamics
Market Growth Drivers
Increased patient assistance programs, increased government initiatives to raise cancer awareness, the rising incidence of breast cancer in Netherlands, strong research and development initiatives from prominent stakeholders, and the rising need for precision medicine are the factors that are fuelling the Netherlands breast cancer therapeutics market expansion.
Market Restraints
The key inhibitor of the Netherlands breast cancer therapeutics market is the patent expiration of certain medications. The expansion of the Netherlands market for breast cancer therapeutics is also hampered by strict laws and regulations, as well as the negative effects of chemotherapy.
Competitive Landscape
Key Players
- Nutricia (NLD)
- Norgine (NLD)
- Rousselot (NLD)
- Oncogenex
- Apthera
- Astellas
- Bionumerik Pharmaceuticals
- Oncothyreon
Healthcare Policies and Regulatory Landscape
In the Netherlands, having a fundamental insurance policy is required. Everyone receives the same package, however, the price that insurers charge may vary by business. The basic insurance coverage for the following year is determined by the government each year. The majority of medical care is subject to a $417.5 minimum deductible under basic insurance. The national health insurance program of the Netherlands provides coverage for breast cancer treatment. Additionally, patients have access to specialized breast cancer clinics, staffed by multidisciplinary teams of medical experts that collaborate to deliver all-inclusive care. The Dutch healthcare system now reimburses mastectomy patients for prosthetic breast implants and reconstructive surgery. The government also pays for sufferers with breast cancer medical expenses and rehabilitation services.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Breast Cancer Therapeutics Segmentation
By Therapy (Revenue, USD Billion):
- Radiation Therapy
- Targeted Therapy
- Ribociclib
- Abemaciclib
- Afinitor
- Everolimus
- Trastuzumab
- Olaparib
- Ado-Trastuzumab Emtansine
- Palbociclib
- Pertuzumab
- Herceptin
- Tykerb (Lapatinib)
- Others
- Targeted Therapy
- Hormonal Therapy
- Selective Estrogen Receptor Modulators (SERMs)
- Aromatase Inhibitors
- Estrogen Receptor Downregulators (ERDs)
- Chemotherapy
- Taxanes
- Anthracyclines
- Anti-metabolites
- Alkylating Agents
- Immunotherapy
By Cancer Type (Revenue, USD Billion):
- Hormone Receptor
- HER+
By Distribution Channel (Revenue, USD Billion):
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































