Netherlands Anti Aging Therapeutics Market Analysis
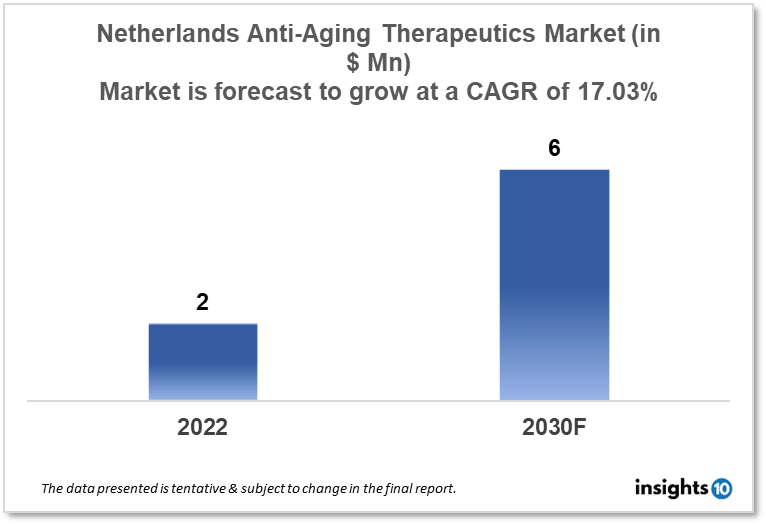
Netherlands anti-aging therapeutics market is projected to grow from $2 Mn in 2022 to $6 Mn in 2030 with a CAGR of 17.03% for the year 2022-2030. Technological advancements in the form of new and inventive therapeutics in the Netherlands as well as increased R&D efforts in the Netherlands are responsible for the market expansion. The Netherlands anti-aging therapeutics market is segmented by product, treatment, target group, type of aging, type of molecules, mechanism of action, ingredient, and by distribution channel. KEI Biotechnology, AHV International, and Biosplice are the major players in the market.
Buy Now

Netherlands Anti-Aging Therapeutics Market Executive Summary
The Netherlands anti-aging therapeutics market size is at around $2 Mn in 2022 and is projected to reach $6 Mn in 2030, exhibiting a CAGR of 17.03% during the forecast period. With a total score of 59.86, the Dutch healthcare system comes in third place in the 2022 World Index of Healthcare Innovation, behind only Switzerland and Ireland. In 2021, the Netherlands placed second, and in 2020, third. The Netherlands performed well overall, finishing no lower than 10th in any category (for Quality). In order to be more prepared for potential pandemics, the Dutch government will place a greater emphasis on international collaboration and strengthening health systems all over the world. Furthermore, the effects of climate change on public health will receive additional attention. Additionally, the Netherlands will increase its spending on foreign health from $116 Mn in 2023 to $143 Mn in 2026.
In the Netherlands, the elderly population has been steadily growing, which has increased the demand for anti-aging health goods to ensure longevity and to lessen age-related complications. Nicotinamide mononucleotide (NMN), one of many anti-aging health products, has caught the interest of both consumers and researchers. Age-related declines in nicotinamide adenine dinucleotide (NAD+) levels are linked to cognitive decline, oxidative stress, DNA damage, and inflammatory diseases. NAD+ is also connected with the downregulation of mitochondrial energy production. However, by raising the body's amount of NAD+, NMN, the precursor to NAD+, can slow this process down. Numerous in vivo studies have shown that NMN supplementation has positive therapeutic benefits for a variety of age-induced complications.
Numerous in vivo studies have revealed that NMN has a broad range of pharmacological activities in addition to its anti-aging potential. There has been significant research on the relationship between NMN and the prevalence of Alzheimer's disease, obesity and its complications, cerebral and cardiac ischemia, and age- and diet-induced type 2 diabetes. Although the scientific community in the Netherlands previously focused on NMN only as an intermediate in the biosynthesis of NAD+, lately a number of pharmacological activities triggered by rising levels of NAD+ in the body, particularly anti-aging activity, have come to the fore.
Market Dynamics
Market Growth Drivers Analysis
The Netherlands is one of many developed nations with an aging population due to a sharp rise in the proportion of individuals 65 and older. The Netherlands anti-aging therapeutics market is expanding as a result of the changing demographics, which has raised the demand for anti-aging products and therapies. New and inventive anti-aging therapies and goods have been designed as a result of technological advancements. Consumers find these new goods more appealing because they are more efficient and come with fewer side effects than conventional treatments. The Dutch government is actively encouraging the growth of the healthcare sector, including the market for anti-aging therapeutics. This assistance comes in the form of grants for R&D and tax breaks for businesses operating in the sector. The Netherlands anti-aging therapeutics market is anticipated to expand as a result of this support.
Market Restraints
In the Netherlands, the regulatory environment for anti-aging products and therapies is complex, which may restrict market development. Production and commercialization of new treatments could be delayed down if companies have trouble getting regulatory approvals for new goods and therapies. The acceptance of anti-aging therapies and goods may be constrained by consumer uncertainty about their efficacy. This might restrict the Netherlands anti-aging therapeutics market expansion, especially among consumers. Some anti-aging treatments and products come with serious risks and side effects, which may discourage customers from using them. This might prevent the market from expanding.
Competitive Landscape
Key Players
- Batavia Biosciences (NLD)
- Barenbrug Holland (NLD)
- Hubrecht Organoid Technology (NLD)
- KEI Biotechnology (NLD)
- AHV International (NLD)
- Biosplice
- Cyclo Therapeutics
- Denali Therapeutics
- Elysium Health
- Genome Protection
- GenSight Biologics
- Intervene Immune
- Khondrian
Healthcare Policies and Regulatory Landscape
The Medicines Evaluation Board (MEB) is the Dutch regulatory authority in charge of regulating and approving anti-aging medications. The Dutch Ministry of Health, Welfare, and Sport oversees the MEB as an independent agency. Its primary duty is to assess and authorize new medications and medical equipment for use in the Netherlands. To receive approval for an anti-aging drug, companies must present an archive of information to the MEB that demonstrates the product's safety, efficacy, and quality. Following an evaluation of the dossier, the MEB will decide whether or not to approve the drug for use in the Netherlands. The MEB also keeps an eye on the security of medicines that are already on the market and has the power to take action if it notices any security problems or issues. If a product is discovered to be unsafe, it may be necessary for businesses to alter the labeling on the product or even remove it from the market.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Anti-Aging Therapeutics Market Segmentation
By Product (Revenue, USD Billion):
- Anti-Wrinkle
- Hair Color
- Ultraviolet (UV) Absorption
- Anti-Stretch Mark
- Others
By Treatment (Revenue, USD Billion):
- Hair Restoration
- Anti-Pigmentation
- Adult Acne Therapy
- Breast Augmentation
- Liposuction
- Chemical Peel
- Others
By Target Group (Revenue, USD Billion):
- Male
- Female
By Type of Aging (Revenue, USD Billion):
- Cellular Aging
- Immune Aging
- Metabolic Aging
- Others
By Type of Molecules (Revenue, USD Billion):
- Biologics
- Small Molecules
By Mechanism of Action (Revenue, USD Billion):
- Senolytic
- Cell Regeneration
- mTOR inhibitor/Modulator
- AMP-kinase/AMP Activator
- Mitochondria Inhibitor/Modulator
- Others
By Ingredient (Revenue, USD Billion):
- Retinoid
- Hyaluronic Acid
- Alpha Hydroxy Acid
- Others
By Distribution Channel (Revenue, USD Billion):
- Pharmacies
- Stores
- Online Stores
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































