Mexico PET Scan Market Analysis
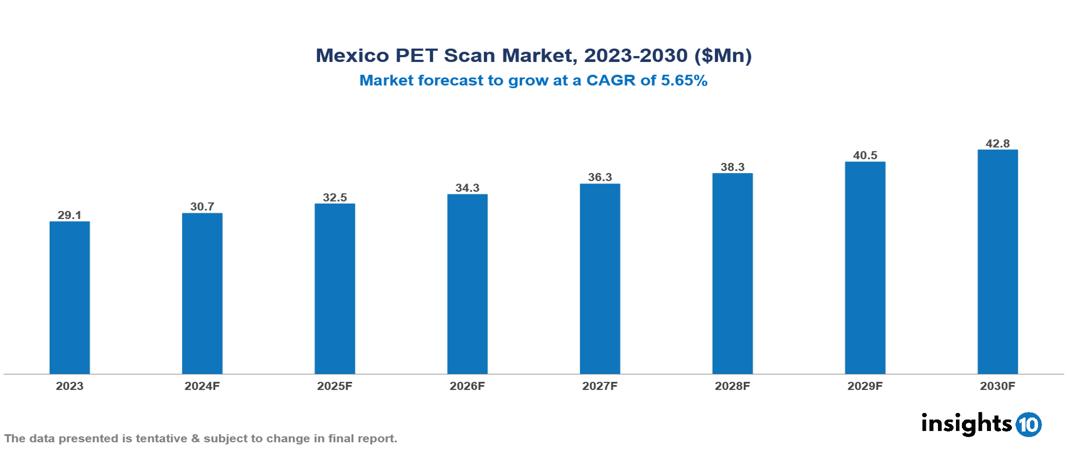
The Mexico PET Scan Market was valued at $29.1 Mn in 2023 and is predicted to grow at a CAGR of 5.65% from 2023 to 2030, to $42.8 Mn by 2030. The key drivers of the market include increased healthcare expenditure, increased geriatric population, and rising prevalence of chronic diseases. The prominent players of the Mexico PET Scan Market are GE Healthcare, Hitachi, Siemens Healthineers, Toshiba, and Canon Medical Systems, among others.
Buy Now

Mexico PET Scan Market Executive Summary
The Mexico PET Scan market is at around $29.1 Mn in 2023 and is projected to reach $42.8 Mn in 2030, exhibiting a CAGR of 5.65% during the forecast period.
PET imaging (Positron Emission Tomography) is a diagnostic imaging tool used in Nuclear Medicine to obtain clinical information from the patient. An extension of this imaging modality is the PET-CT hybrid. The system consists of two scanning machines: PET imaging and an X-ray computed tomography (CT) scanner that are combined in a PET-CT scan to provide detailed information on the anatomy and physiology of the body. It is usually performed in a single scanning session and is an established technology which is widespread and accepted globally. A CT scan is a diagnostic imaging procedure that uses a combination of X-rays and computer technology to produce images of the inside of the body. A PET-CT scan is a hybrid imaging method that combines the anatomical and functional imaging of CT with PET to produce finely detailed, high-resolution pictures for use in the diagnosis and treatment of a variety of medical disorders. Another frequently used integrated diagnostic imaging method is PET-MRI scan. MRI is magnetic resonance imaging which uses strong magnetic fields and radio waves to produce finely detailed images of the brain and other soft tissues like muscles and ligaments. PET-MRI scan provides superior soft tissue contrast compared to CT scans in PET-CT which allows for more precise anatomical visualization alongside the functional data from the PET scan. Moreover, PET-MRI offers sharper images than CT scans, particularly in regions close to bones, which can introduce artifacts because of bone density. When identifying disorders of the pelvis, spine, or skull, this can be extremely important.
Chronic diseases are a significant healthcare burden in Mexico, contributing to high morbidity and mortality rates and placing considerable pressure on the healthcare system. The Mexico PET Scan Market is thus driven significant factors such as the increased healthcare expenditure, increased geriatric population, and rising prevalence of chronic diseases. However, the radiation exposure concerns, regulatory challenges, and lack of skilled professionals restrict the growth and potential of the market.
The major players of the Mexico PET Scan Market are GE Healthcare, Hitachi, Siemens Healthineers, Toshiba, and Canon Medical Systems, among others.
Market Dynamics
Market Growth Drivers
Increased Healthcare Expenditure: Increased healthcare expenditure facilitates the expansion and modernization of healthcare infrastructure, prompting new hospitals, diagnostic centres, and specialty clinics to integrate PET scanning services. This enhances their diagnostic capabilities and attracts patients seeking advanced medical care. Moreover, healthcare systems with higher expenditures emphasize integrated care approaches, leveraging PET scans alongside other imaging technologies like CT or MRI for comprehensive diagnostic insights. This integration enhances treatment planning and management, driving market growth. Additionally, increased spending supports research and development, with PET scans playing a crucial role in clinical trials, drug development, and medical research, thereby advancing PET scan technology and expanding its clinical applications.
Increasing Geriatric Population: In Mexico, 9% of the total population was covered by individuals aged 65 and above in 2023. As individuals age, their susceptibility to various age-related ailments, notably chronic conditions such as cancer, cardiovascular diseases, and neurodegenerative disorders, increases. PET scans are instrumental in diagnosing and monitoring these conditions, creating heightened demand among older populations. Consequently, older adults often present complex medical requirements that necessitate precise and comprehensive diagnostic tools. By providing detailed insights into disease progression, treatment effectiveness, and overall health management, PET scans facilitate personalized care strategies. Overall, the aging demographic underscores the necessity for PET scans, thereby stimulating growth in the PET scan market.
Rising Prevalence of Chronic Diseases: The increasing incidence of chronic diseases like cancer, cardiovascular disorders, and neurodegenerative conditions underscores the essential need for advanced diagnostic tools such as PET scans. To support this, according to the WHO Global Cancer Observatory, the age standardized cancer incidence rate was 141.3 and 141.8 in males and females, respectively in 2022. PET scan is crucial for precisely detecting, staging, planning treatments, and monitoring these ailments from an early stage. The advantages offered by PET scan drive the market growth.
Market Restraints
Radiation Exposure Concerns: PET scans offer significant medical benefits, but radiation exposure is a notable concern affecting patient safety, public perception, and regulatory compliance. The use of radiotracers emitting ionizing radiation, even in low doses, poses potential risks with repeated exposure, leading to patient hesitation. Pregnant women and children are particularly sensitive to radiation, restricting PET scan use in these populations. These concerns impact the overall growth of the PET scan market.
Regulatory Challenges: The regulatory approval process for radiotracers used in PET scans is stringent, requiring extensive preclinical research, clinical trials, and regulatory filings. Delays in licensing can hinder the introduction of novel radiotracers and advanced imaging options. Additionally, regional and national regulatory constraints complicate the introduction of innovative PET imaging technologies, further delaying market entry and adoption. Consequently, the overall regulatory approval process for medical devices can be a significant market restraint for the PET scan market.
Lack of Skilled Professionals: A significant impediment to the PET Scan Market is the lack of proficient personnel with training in PET technology. Extensive training and experience are necessary for operating PET scanners and correctly analyzing the complicated data they output. Lack of such knowledge can impede the growth of PET scan services, especially in areas where access to specialist training programs is restricted.
Regulatory Landscape and Reimbursement Scenario
In Mexico, the Federal Commission for Protection against Health Risks (COFEPRIS or Comisión Federal para la Protección contra Riesgos Sanitarios) is a regulatory body of the Mexican Government; a decentralized organ of the Mexican Secretariat of Health. Among the many health-related issues it regulates in Mexico are pharmaceuticals, food safety, medical devices, organ transplants, and environmental preservation. COFEPRIS is in charge of the entire drug approval process, from the initial application to the post-market surveillance (PMS) stage, including supply and demand, advertising, commercialization, imports and exports, distribution, manufacturing, and supply.
Mexico’s pharmaceutical industry is thriving and expanding, and thus strict laws are in place to guarantee the security and effectiveness of medications made available to Mexican residents. Pharmaceutical companies seeking to sell their medications in Mexico must understand the procedure thoroughly for getting approval. The overall approval includes an extensive evaluation by COFEPRIS, accompanied by facility inspection to ensure GMP compliance. There are three different pathways for registering medicinal product with COFEPRIS. The New Drug Registration is the pathway applicable for new chemical entities that have not previously marketed anywhere in the world. Next, is the Generic Drug Registration for drugs that are similar to the already-marketed reference drugs. Lasty, Biosimilar Registration is applicable to the biological products that are similar to the approved reference biological product.
The healthcare reimbursement system in Mexico is characterized by a complex interplay between public and private sectors. For the public sector, the social security institutions are the main provider of public health insurance. Workers in the formal sector and their dependents are covered by the Instituto Mexicano del Seguro Social (IMSS). Government employees and their families are covered by the Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado (ISSSTE). To supplement public plans with extra coverage, many Mexicans opt to buy private health insurance which provides access to a greater variety of healthcare providers, such as private hospitals and specialists. The healthcare reimbursement scheme in Mexico is gradually evolving. Although many people have a safety net in public services, there are still large differences. In the future, there may be initiatives to increase public-private partnership, achieve universal coverage, and use technology to improve healthcare affordability and accessibility for all.
Competitive Landscape
Key Players
Here are some of the major key players in the Mexico PET Scan Market:
- GE Healthcare
- Hitachi
- Siemens Healthineers
- Toshiba
- Canon Medical Systems
- Hermes Medical Solutions
- Philips Healthcare
- Mediso Medical Imaging Systems
- Shimadzu Corporation
- Neusoft Medical Systems
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Mexico PET Scan Market Segmentation:
By Modality
- PET Standalone Scanners
- PET-CT Scanners
- PET-MRI Scanners
By Application
- Oncology
- Cardiology
- Neurology
- Other
By End-User
- Hospitals and Surgical Centers
- Diagnostic and Imaging Clinics
- Ambulatory Surgical Centers
- Research Institutes
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.









































