Mexico Neurology Devices Market Analysis
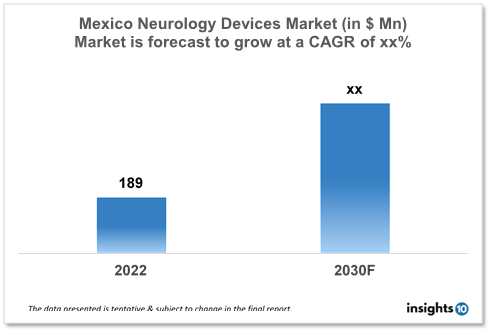
The Mexico Neurology Device Market is expected to witness growth from $189 Mn in 2022 to $xx Mn in 2030 with a CAGR of xx% for the forecasted year 2022-2030. Mexico's population is quickly ageing, with an increase in the proportion of people over 60. Age increases a person's vulnerability to brain diseases like Alzheimer's, Parkinson's, and stroke. The demand for neurology devices used in the diagnosis and treatment of these disorders, such as neurostimulation devices and imaging systems, is anticipated to increase as a result of this demographic tendency. The market is segmented by product type and by the end user. Some key players in this market include Johnson and Johnson, GE Healthcare, Stryker, Medtronic, Abbott Laboratories and Philips Healthcare.
Buy Now

Mexico Neurology Devices Market Executive Analysis
The Mexico Neurology Devices Market size is at around $189 Mn in 2022 and is projected to reach $xx Mn in 2030, exhibiting a CAGR of xx% during the forecast period. Health spending in Mexico will grow significantly as a percentage of GDP, from 5.4% in 2019 to 6.2% in 2020 (in contrast to an increase of 0.9 % on average in the Organization for Economic Cooperation and Development region). Mexico spent 6.2% of its Gross Domestic Product on healthcare in 2020. (GDP). The Newborn mortality rate in Mexico is 13 per 1,000 live births, compared to an average of 3.8 in the Organization for Economic Cooperation and Development (OECD). Although the percentage of out-of-pocket expenses in Mexico has dropped from 55% to 45%, the populace still bears the burden.
In Mexico, epilepsy is a common brain condition. Epilepsy affects roughly 2.5 million individuals in Mexico, with a prevalence of 9 cases per 1,000 people. In Mexico, stroke is the main source of death and disability. About 60,000 stroke-related fatalities occurred in Mexico in 2020. In Mexico, there are about 70 cases of stroke for every 100,000 individuals. Parkinson's disease is a prevalent neurodegenerative disorder in Mexico, where there are 200 cases of the disease per 100,000 individuals. In Mexico, multiple sclerosis is a relatively uncommon neurological condition; there are approximately 5.1 cases of multiple sclerosis per 100,000 individuals in Mexico. Neurological disorders like epilepsy and Parkinson's disease are treated with the aid of neurology devices. Parkinson's disease can be treated with Deep Brain Stimulation (DBS), which entails implanting a device that transmits electrical impulses to the brain to lessen tremors and other motor symptoms. Another option for treating epilepsy is Vagus Nerve Stimulation (VNS), which entails implanting a device that transmits electrical impulses to the vagus nerve to lessen seizures.
Market Dynamics
Market Growth Drivers
Mexico's population is quickly ageing, with an increase in the proportion of people over 60. Age increases a person's vulnerability to brain diseases like Alzheimer's, Parkinson's, and stroke. The demand for neurology devices used in the diagnosis and treatment of these disorders, such as neurostimulation devices and imaging systems, is anticipated to increase as a result of this demographic tendency. The creation of sophisticated neurostimulation devices and imaging systems, among other technological developments, have improved the accuracy and efficacy of the diagnosis and treatment of neurological disorders. As a result of healthcare practitioners wanting to provide their patients with the most up-to-date and efficient treatments possible, this is anticipated to increase demand for advanced neurology devices in Mexico.
Market Restraints
Mexico's stringent payment regulations for neurology devices may deter medical professionals from implementing cutting-edge neurology equipment. To create reimbursement guidelines that encourage the use of their goods, manufacturers may need to collaborate with healthcare professionals and insurers. The market for neurology products in Mexico can be severely constrained by regulatory obstacles. The lengthy and complicated regulatory procedure may delay market entry and raise costs. To successfully navigate these obstacles, manufacturers may need to engage in regulatory expertise.
Competitive Landscape
Key Players
- Philips Healthcare
- Medtronic
- Abbott Laboratories
- Johnson & Johnson
- Boston Scientific
- Nihon Kohden
- GE Healthcare
- Stryker
Notable Recent Deals
October 2020: Leading medical device company Medtronic proclaimed in October 2020 that it had teamed up with Diabetic Foot Care México to sell its neurology devices in Mexico. In order to cure neurological disorders, the partnership will concentrate on distributing Medtronic's Deep Brain Stimulation (DBS) and Spinal Cord Stimulation (SCS) systems.
Healthcare Policies and Regulatory Landscape
Private and public healthcare organisations coexist in the Mexican healthcare system. The Federal Commission for Protection Against Sanitary Risks (COFEPRIS), an agency of the Ministry of Health, is in charge of regulating neurology devices in Mexico. Neurology devices must adhere to COFEPRIS's Mexican Official Standards in order to operate legally in Mexico. Manufacturers of neurology devices must receive COFEPRIS clearance before they can market their devices in Mexico. The safety, effectiveness, and conformance to regulatory requirements of the device are all examined as part of the approval procedure.
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Neurology Device Market Segmentation
The Neurology Device Market is segmented as mentioned below:
By Product Type (Revenue, USD Billion):
- Neurostimulation
- ?Spinal Cord Stimulation Devices
- Deep Brain Stimulation Devices
- Sacral Nerve Stimulation
- Vagus Nerve Stimulation
- Gastric Electric Stimulation
- Interventional Neurology
- Aneurysm Coiling & Embolization
- Embolic Coils
- Flow Diversion Devices
- Liquid Embolic Agents
- Cerebral Balloon Angioplasty & Stenting
- Carotid Artery Stents
- Filter Devices
- Balloon Occlusion Devices
- Neurothrombectomy
- Clot Retriever
- Suction Aspiration Devices
- Snares
- CSF Management
- CSF Shunts
- CSF Drainage
- Neurosurgery Devices
- Ultrasonic Aspirators
- Stereotactic Systems
- Neuroendoscopes
- Aneurysm Clips
By End User (Revenue, USD Billion):
- ??Hospitals and Clinics
- Specialty Centres
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































