Mexico Medication Access Programs Market Analysis
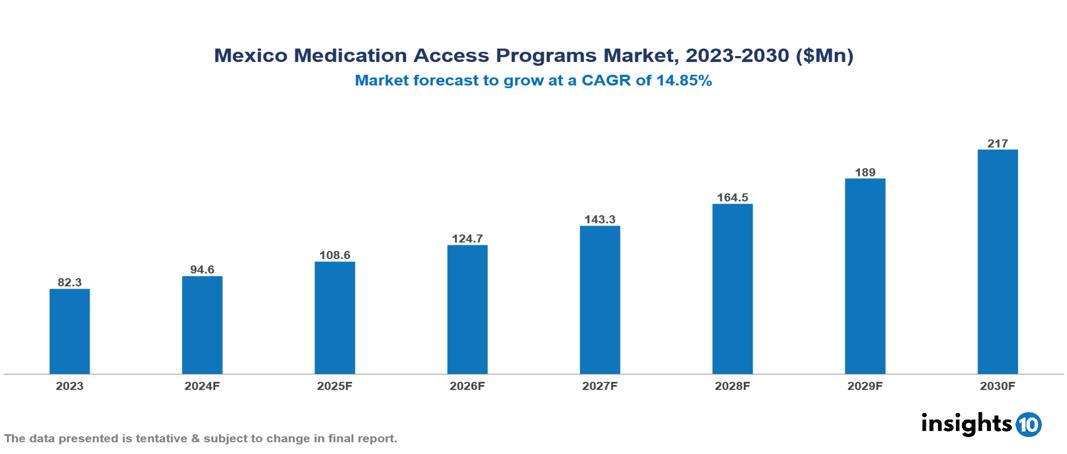
The Mexico Medication Access Programs Market was valued at $82.3 Mn in 2023 and is predicted to grow at a CAGR of 14.85% from 2023 to 2030, to $217 Mn by 2030. The key drivers of this industry include healthcare reforms, disease burden, and government support. The industry is primarily dominated by players such as Gilead Sciences, Takeda Pharmaceuticals, Pfizer, and Novartis among others.
Buy Now

Mexico Medication Access Programs Market Executive Summary
The Mexico Medication Access Programs Market was valued at $82.3 Mn in 2023 and is predicted to grow at a CAGR of 14.85% from 2023 to 2030, to $217 Mn by 2030.
Patient Support Programs (PSPs) are initiatives organized by pharmaceutical companies to enhance access, usage, and adherence to prescription drugs. These programs encompass financial assistance, clinical support, educational efforts, or a blend of these elements. A Medication Access Program (MAP) is a part of PSP that serves as a crucial connection between patients and the medications they need. Managed Access Programs are initiatives that enable patients with serious or life-threatening illnesses to obtain investigational medicines or treatments not yet commercially available. These programs aim to provide early access to therapies for patients who have exhausted all other options and are ineligible for clinical trials. Offered by pharmaceutical companies, MAPs help patients overcome financial barriers to access essential medications. A centralized, pharmacy-driven MAP can enhance patient outcomes, reduce unnecessary healthcare costs, improve patient and provider satisfaction, streamline patient flow, and boost revenue through increased prescription capture.
Research has identified ongoing challenges with the availability, distribution, and affordability of medications, especially within the public healthcare system. Among users of the Mexican Institute of Social Security (IMSS), 86% who received prescriptions successfully obtained all their medications, whereas this figure was 63% for users of Seguro Popular (public insurance). The market is driven by significant factors like healthcare reforms, disease burden, and government support. However, budgetary constraints, regulatory hurdles, and supply chain issues restrict the growth and potential of the market.
Prominent players in this field include Gilead Sciences and Takeda Pharmaceuticals which provide Medication Access or Access to Medicine Program. Pfizer, Novartis, Merck, and AstraZeneca among others are some of the pharmaceutical companies providing patient support programs and are potential players for the Medication Access Program in Mexico.
Market Dynamics
Market Growth Drivers
Healthcare Reforms: Healthcare reforms aimed at improving access to medications and healthcare services across all population segments are a market driver for Medication Access Programs in Mexico. These reforms seek to broaden access to essential treatments, creating opportunities for MAPs to play a crucial role in ensuring patients have timely and affordable access to necessary medications, thereby driving the growth and effectiveness of MAPs in the country.
Disease Burden: In 2021, Mexico had an incidence of 17 new cases of tuberculosis per 100,000 people. Additionally, in 2023, 11.9% of individuals aged 15 and older in Mexico used tobacco. These statistics serve as market drivers for the Medication Access Program market in Mexico because they highlight significant public health challenges that require effective medication access and management programs.
Government Support: Government initiatives and policies that subsidize medications or support patient assistance programs drive the Medication Access Program (MAP) market in Mexico by reducing financial barriers to medication access, thereby enhancing availability and affordability for patients nationwide. These efforts are crucial in expanding MAPs and improving healthcare access and outcomes across the country.
Market Restraints
Budgetary Constraints: Constraints in healthcare funding can limit the financial resources available for Medication Access Programs, thereby restricting their implementation and reach. This acts as a market restraint in Mexico because inadequate funding hinders the ability of MAPs to effectively provide medications and support services, thereby limiting their impact on improving healthcare access and outcomes for the population.
Regulatory Hurdles: The complexity of regulatory processes and bureaucratic challenges can delay the approval and distribution of medications through Medication Access Programs, thereby affecting timely access for patients. This serves as a market restraint in Mexico because these obstacles hinder the efficient operation of MAPs, potentially delaying or limiting patients' ability to obtain essential medications promptly and efficiently.
Supply Chain Issues: Supply chain disruptions, like shortages or delays in drug procurement, can compromise the reliability of medications provided through Medication Access Programs (MAPs), acting as a market restraint in Mexico by hindering timely access for patients.
Regulatory Landscape and Reimbursement Scenario
In Mexico, oversight of pharmaceuticals, medical devices, and national health policy falls under the jurisdiction of the Secretariat of Health. The Federal Committee for Protection from Sanitary Risks or Comisión Federal para la Protección contra Riesgos Sanitarios (COFEPRIS) is responsible for regulating healthcare facilities, overseeing advertising, and managing the production, importation, and exportation of medical supplies. The healthcare system in Mexico is characterized by a combination of public and private sectors. Public healthcare services are administered by organizations such as the Mexican Institute of Social Security or Instituto Mexico de Seguro Social (IMSS), the Institute of Safety and Social Services for Public Sector Workers or Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado (ISSSTE), and the Secretary of Health or Secretaria de Salud (SSA). The private sector includes clinics, hospitals, and other healthcare facilities.
Competitive Landscape
Key Players
Here are some of the major key players in the Mexico Medication Access Programs Market:
- Gilead Sciences
- Takeda Pharmaceuticals
- Pfizer
- Novartis
- Merck
- AstraZeneca
- Bristol-Myers Squibb
- Sanofi
- Eli Lilly
- AbbVie
1. Executive Summary
1.1 Service Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Healthcare Services Market in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Services
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Mexico Medication Access Programs Market Segmentation
By Disease Type
- Chronic
- Acute
By Therapeutic Areas
- Oncology
- Cardiology
- Rheumatology
- Others
By Patient Type
- Geriatric
- Pediatric
- Adult
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.









































