Mexico Hepatitis A Therapeutics Market Analysis
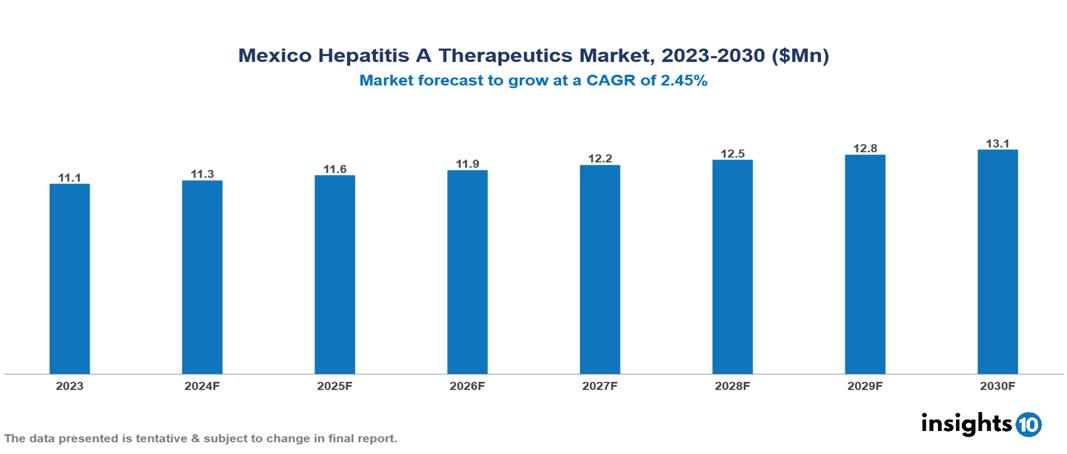
The Mexico Hepatitis A Therapeutics Market was valued at $11.06 Mn in 2023 and is predicted to grow at a CAGR of 2.45% from 2023 to 2030 to $13.1 Mn by 2030. Factors including growing prevalence, an aging population, and personal hygiene and sanitation challenges propel the market. Leading companies in this sector include F. Hoffmann-La Roche Ltd. and Merck & Co. Inc., among others.
Buy Now

Mexico Hepatitis A Therapeutics Market Executive Summary
The Mexico Hepatitis A Therapeutics Market was valued at $11.06 Mn in 2023 and is predicted to grow at a CAGR of 2.45% from 2023 to 2030 to $13.10 Mn by 2030.
Hepatitis A is a contagious liver disease typically transmitted through contaminated food or water or close personal contact with an infected individual, presenting with varying severity from mild illness lasting weeks to more severe complications lasting months. It is notably prevalent in Mexico, with significant seroprevalence rates across various age groups. The increasing incidence, particularly in regions with inadequate sanitation and limited access to clean water, stimulates demand for treatments and preventive measures like vaccination programs. Mexico's aging population heightens susceptibility to Hepatitis A infections, posing considerable health risks. Addressing personal hygiene and sanitation challenges is pivotal in mitigating the spread of Hepatitis A and lessening its public health impact in Mexico.
Mexico has a high prevalence of Hepatitis A, with an average seroprevalence of 69.3%, ranging from 58.8% in adolescents to 83% in young adults, making Hepatitis A the primary cause of viral hepatitis in the general population and the most common viral infection among children. Factors including growing prevalence, an aging population, and personal hygiene and sanitation challenges propel the market, while factors like lack of healthcare infrastructure, high cost, and limited inclusion in national immunization programs restrain the market.
Market Dynamics
Market Growth Drivers
Rising Prevalence: Hepatitis A is increasingly common in Mexico, emerging as the primary cause of viral hepatitis in the general population (79%) and the most prevalent viral infection among children. The seroprevalence of Hepatitis A in Mexico is significant, with an average weighted HAV (Hepatitis A Virus) seroprevalence of 69.3%, varying from 58.8% in adolescents to 83.0% in young adults. This widespread prevalence highlights the significant impact of Hepatitis A across the country. The growing incidence of Hepatitis A infections in Mexico, particularly in regions with inadequate sanitation and limited access to clean water, is spurring demand for effective treatments and preventive measures such as vaccination programs and enhanced healthcare strategies.
Aging Population: Mexico's aging population is increasingly susceptible to Hepatitis A infections, with anti-HAV seropositivity reaching 96.9% in adults aged ≥ 20 years. As the population ages, the number of vulnerable individuals is rising, posing substantial health risks, including liver failure and mortality. Recent data suggests a shift in the age groups affected by Hepatitis A, with a declining incidence trend and a notable increase observed during 2017-2019. This indicates that as Mexico's population continues to age, the burden of Hepatitis A is likely to escalate among older adults, emphasizing the need for targeted therapeutics and preventive measures, thus driving the market.
Poor Hygiene and Sanitation Practices: Inadequate personal hygiene and sanitation practices significantly contribute to the demand for Hepatitis A therapeutics in Mexico. Issues related to water disinfection, lack of proper handwashing facilities, and inadequate wastewater disposal impact hygiene practices, with many households failing to disinfect drinking water and lacking appropriate handwashing facilities. This creates opportunities for pharmaceutical companies and healthcare providers to cater to the needs of affected populations with effective treatments and preventative measures.
Market Restraints
Lack of healthcare infrastructure: The limited availability of healthcare facilities equipped to handle liver diseases, such as liver transplants, in Mexico hinders the growth of the Hepatitis A therapeutics market. Investments in healthcare infrastructure are necessary to support the effective management of Hepatitis A and other liver diseases.
High Cost: Hepatitis A vaccines, ranging from $26 to $66 per dose for uninsured adults and $40 to $58 per dose at private healthcare facilities in Mexico, are a significant barrier to their widespread adoption. Compared to other childhood immunizations, this high cost hinders the inclusion of Hepatitis A vaccines in Mexico's National Immunization Program. The relatively high price of Hepatitis A vaccines limits access to the vaccines and restrains the market growth for Hepatitis A therapeutics in Mexico.
Limited Inclusion in National Immunization Programs: The lack of inclusion of Hepatitis A vaccines in Mexico's National Immunization Program limits access and uptake of the vaccine, impacting the market for Hepatitis A therapeutics.
Regulatory Landscape and Reimbursement Scenario
The reimbursement scenario for Hepatitis A therapeutics in Mexico is influenced by various factors, including patient situation, payer type, specific medications used, and cost-effectiveness considerations. Regulatory bodies like COFEPRIS (Comision Federal para la Proteccion contra Riesgos Sanitarios) and the Secretaría de Salud (Mexican Secretariat of Health) oversee drug approvals, registration, and compliance with health regulations, ensuring compliance with regulatory standards and safeguarding public health.
Public payers like Instituto Mexicano del Seguro Social (IMSS) and Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado (ISSSTE) may cover Hepatitis A treatments for high-risk individuals, such as unvaccinated or incompletely vaccinated patients with clinically significant infections, immunocompromised individuals, and those needing post-exposure prophylaxis, subject to prior authorization. Private health insurance coverage varies based on plan details and formularies, with some offering broader coverage and others having stricter criteria. The limited number of approved Hepatitis A medications and Mexico's focus on vaccination may impact reimbursement decisions.
Competitive Landscape
Key players
Here are some of the major key players in the Hepatitis A Therapeutics Market:
- F. Hoffmann-La Roche Ltd.
- Merck & Co. Inc.
- Zydus Cadilla
- Sanofi
- GlaxoSmithKline (GSK)
- Takeda
- CVS Health Corporation
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Mexico Hepatitis A Therapeutics Market Segmentation
By Distribution Channel
- Hospital-based pharmacies
- Retail pharmacies
- Online pharmacies
By Route of Administration
- Oral Medications
- Intravenous Therapy
By Healthcare Setting
- Outpatient Care
- Inpatient Care
By Age
- Children
- Adults
- Senior Citizens
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.









































