Mexico Dermatological Therapeutics Market Analysis
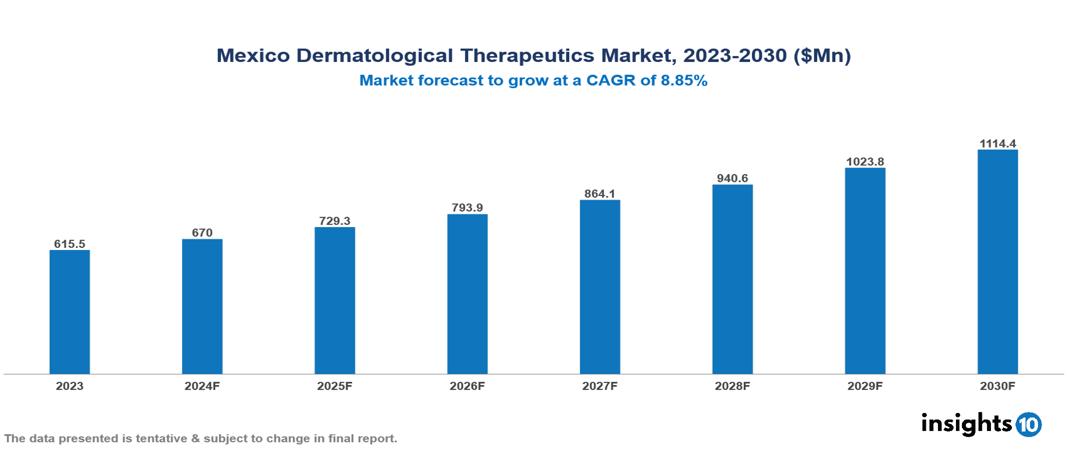
Mexico Dermatological Therapeutics Market is at around $0.62 Bn in 2022 and is projected to reach $1.11 Bn in 2030, exhibiting a CAGR of 8.85% during the forecast period. The market is rapidly expanding as a result of the increased prevalence of dermatological conditions, rising awareness about skin disorders, and technological advancements in the industry. The market is dominated by key players like Genomma Lab Internacional, Almirall SA, Pfizer Inc., AbbVie Inc., Amgen, Gladerma Laboratories, Bausch Health Companies Inc., Novartis AG, Eli Lily and Company, and GlaxoSmithKline PLC.
Buy Now

Mexico Dermatological Therapeutics Market Executive Summary
Mexico Dermatological Therapeutics Market is at around $0.62 Bn in 2023 and is projected to reach $1.11 Bn in 2030, exhibiting a CAGR of 8.85% during the forecast period.
Mexico's market for dermatological therapeutics includes pharmaceutical and therapeutic items intended for use in the country's healthcare system to treat a range of skin ailments. Dermatological medications and therapies, which address a wide range of skin-related disorders from acne and eczema to more complicated dermatological conditions, are developed, distributed, and utilized in this active sector. The market for dermatological therapeutics is essential in meeting Mexico's expanding need for dermatological care by offering cutting-edge skincare solutions that successfully address the country's need for these treatments.
The growing prevalence of dermatological disorders is fueling the steady growth of the Mexico Dermatological Therapeutics Market. The need for dermatological treatments is fueled by increased skincare awareness. To satisfy the changing demands of consumers in the Mexican dermatological treatments sector, major players in the market are concentrating on innovation and product development.
With $40.94 Bn in revenue, the global market for dermatological treatments had an incredible rise in 2023. Modern technology and highly effective production techniques enable this enormous expansion, which is a sign of a shifting society. More investment, continuous innovation, and increased accessibility are all contributing to the industry's rapid growth. The market for dermatological treatments has benefited from these factors, which has also established the industry's growth.
Genomma Lab International is a significant competitor in the Mexican dermatological therapeutics industry, with a projected 25% of market share as of 2023 and showcasing a robust portfolio of more than 40 skin health-related brands. They face competition against established multinational companies like AbbVie and Amgen, but Genomma has an advantage due to its well-known brand and robust local presence.
Market Dynamics
Market Growth Drivers:
Increasing Dermatological Conditions: The need for dermatological treatments such as psoriasis, eczema, acne, and skin cancer is rising as the prevalence of these conditions increases, which is driving the market.
Increasing Healthcare Awareness: People may seek medical attention more frequently as a result of improved skincare knowledge and the accessibility of cutting-edge dermatological therapies, which is growing the market for dermatological therapeutics.
Technological Developments: The market may expand as a result of improvements in medication formulations and dermatological treatment technology. Patients and healthcare professionals may be drawn to new and improved products with greater efficacy and fewer adverse effects.
Market Restraints:
Regulatory Obstacles: Extensive approval procedures and strict regulations about dermatological medications and treatments may impede the growth of the market. The timely introduction of novel medicines might be impeded by delays in product approvals and regulatory compliance.
Competition from Alternative Therapies: The market share of traditional dermatological treatments may be impacted by competition from alternative therapies, which may be seen as more natural or culturally acceptable.
Limited Healthcare Infrastructure: The availability of dermatology clinics and specialized treatment facilities may be restricted in some areas of Mexico. This may limit the availability of dermatological treatments, especially in remote locations.
Healthcare Policies and Regulatory Landscape
Secretaría de Salud, or Mexican Secretariat of Health, is the agency in charge of managing laws about pharmaceuticals and medical devices as well as other aspects of health services, such as national health policy. To supervise the process, the Comisión Federal para la Protección contra Riesgos Sanitarios (COFEPRIS) collaborates with the Sanitary Authorization Commission (CAS), which is in charge of sanitary registration of medications in Mexico. The COFEPRIS New Molecule Committee handles requests and technical meetings through the Center for Integrated Services (Centro Integral de Servicios, CIS). The Mexican government rigorously controls the price of medications and places a high premium on healthcare accessibility. For new and expensive drugs, this can be a major obstacle that makes it hard for companies to recoup their initial investment.
Competitive Landscape
Key Players:
- Genomma Lab Internacional
- Almirall SA
- Pfizer Inc.
- AbbVie Inc.
- Amgen Inc.
- Gladerma Laboratories
- Bausch Health Companies Inc.
- Novartis AG
- Eli Lily and Company
- GlaxoSmithKline PLC
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Mexico Dermatological Therapeutics Market Segmentation
By Type
- Prescription-based Drugs
- Over-the-counter Drugs
By Disease
- Alopecia
- Herpes
- Psoriasis
- Rosacea
- Skin Cancer
- Acne
- Atopic Dermatitis
- Vitiligo
- Hidradenitis
- Other Applications
By Drug Class
- Anti-infectives
- Corticosteroids
- Anti-acne
- Calcineurin Inhibitors
- Retinoids
- Other Drug Classes
By Route of Administration
- Topical Administration
- Oral Administration
- Parenteral Administration
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































