Mexico Constipation Therapeutics Market Analysis
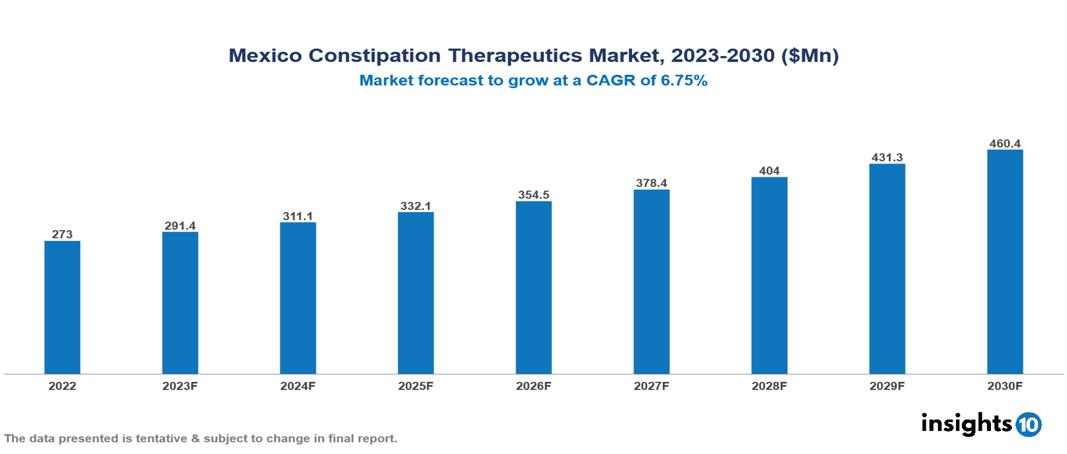
Mexico Constipation Therapeutics Market was valued at $273 Mn in 2022 and is estimated to reach $460 Mn in 2030, exhibiting a CAGR of 6.75% during the forecast period. The constipation therapeutics market is experiencing growth driven by rising global rates of constipation associated with factors like aging, sedentary lifestyles, and poor dietary habits, as well as the expanding elderly population, which is particularly prone to chronic conditions. Notable participants in this industry include AbbVie, Allergan, Boehringer Ingelheim, EMS, Farmoquímica, Grünenthal, Johnson & Johnson, Norgine, Pfizer, and Sanofi.
Buy Now

Mexico Constipation Therapeutics Market Executive Summary
Mexico Constipation Therapeutics Market was valued at $273 Mn in 2022 and is estimated to reach $460 Mn in 2030, exhibiting a CAGR of 6.75% during the forecast period.
Constipation is a digestive condition characterized by irregular bowel movements, difficulty in stool passage, or the discharge of hard and dry stool. This condition arises when the transit of stool through the colon (large intestine) slows down, resulting in increased water absorption and stool hardening. Typical symptoms of constipation include straining during bowel movements, a feeling of incomplete evacuation, abdominal discomfort, and irregular or infrequent bowel habits. Multiple factors, such as a low-fiber diet, insufficient fluid intake, lack of physical activity, specific medications, and underlying medical issues, can contribute to constipation. Addressing constipation often involves lifestyle modifications, dietary changes, increased physical activity, and, when necessary, the use of medications to manage and alleviate symptoms.
Constipation prevalence ranges from 2.4% to 22.3% in Mexico, with an average of 14.4%. Constipation affects women more frequently than it does men. There may be potential peaks in the prevalence of certain population groups, such as children and adults over 65. Furthermore, there are higher rates of constipation in rural areas than in urban areas, which could be associated with factors like dietary variations or restricted access to healthcare.
In the realm of constipation therapeutics, promising advancements are being made through the targeting of specific receptors. Clinical trials indicate the potential efficacy of drugs focusing on guanylate cyclase C (GC-C) and serotonin 4 (5-HT4) receptors. These targeted approaches aim to enhance gastrointestinal function and alleviate constipation symptoms. Moreover, ongoing research is delving into innovative targets such as bile acid signaling and modulation of the enteric nervous system. The exploration of these novel avenues underscores a dynamic and evolving landscape in the development of constipation treatments, offering potential breakthroughs in addressing underlying causes and improving patient outcomes.
Market Dynamics
Market Growth Drivers
Increase in Elderly Population: The growing elderly population in Mexico is a significant driver for the constipation therapeutics market. As people age, they are more prone to constipation, leading to a higher demand for constipation treatment. According to a report, more than half of individuals over 50 are susceptible to persistent constipation, and constipation affects roughly 16% of adults worldwide, with a higher prevalence in the elderly.
Changing Dietary Habits and Sedentary Lifestyles: The changing dietary habits and sedentary lifestyles in Mexico have contributed to an increased demand for constipation treatment. Factors such as insufficient consumption of fast food, low fiber intake, and reduced physical activity have been associated with constipation. The COVID-19 pandemic and the associated lockdown measures have also led to the development of new-onset constipation symptoms, highlighting the impact of lifestyle changes on digestive health.
Improvements in Healthcare Infrastructure: The improvements in healthcare infrastructure in Mexico have led to better diagnosis and treatment of constipation, contributing to market growth. The presence of healthcare professionals, the high prevalence of irritable bowel syndrome (IBS) and chronic constipation, and the development of advanced drugs and treatment procedures have revolutionized the constipation treatment market.
Market Restraints
Regulatory Environment: The regulatory environment presents challenges for the constipation therapeutics market. The prolonged and costly process of obtaining approval for new medications delays their market availability while navigating intricate reimbursement procedures can impede access for both healthcare institutions and patients. This regulatory landscape acts as a significant restraint, affecting the timely introduction and accessibility of specific constipation medications in the market.
Cost and Affordability: Cost and affordability pose significant challenges for the constipation therapeutics market. The high expenses associated with newer medications limit accessibility, particularly for those lacking comprehensive insurance coverage. Unequal access to healthcare, especially in rural areas, exacerbates the issue, hindering timely diagnosis and treatment of constipation. Additionally, even with insurance, elevated out-of-pocket costs for specific medications or laxatives act as barriers for some patients, impacting market penetration and growth.
Sociocultural Factors: Sociocultural factors, including constipation stigma and reliance on traditional remedies, pose a significant restraint on the constipation therapeutics market. The stigma leads to hesitancy in seeking medical assistance, while the preference for traditional remedies can cause delays in proper diagnosis and treatment. Overcoming these barriers requires tailored educational initiatives and the development of therapeutics aligned with local practices to enhance their effectiveness in the market.
Healthcare Policies and Regulatory Landscape
In Mexico, the oversight of healthcare policies and regulatory control over therapeutic drugs primarily falls under the Federal Committee for Protection against Sanitary Risk (COFEPRIS), a branch of the Ministry of Health. COFEPRIS collaborates with the Ministry of Health to ensure the effectiveness, safety, and quality of medications. The National Cancer Institute (INCan) serves as a key reference organization for cancer care, and the National Institute of Public Health (INSP) contributes to the scientific foundation for healthcare decisions. The Mexican Social Security Institute (IMSS) plays a role in influencing drug accessibility and usage regulations for insured individuals, while the General Health Council provides advisory services on health-related matters. Additionally, the National Commission for Protection against Health Risks (CONARIS) facilitates risk evaluation and management.
Competitive Landscape
Key Players
- AbbVie
- Allergan
- Boehringer Ingelheim
- EMS
- Farmoquímica
- Grünenthal
- Johnson & Johnson
- Norgine
- Pfizer
- Sanofi
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Mexico Constipation Therapeutics Market Segmentation
By Therapeutic
- Laxatives
- Chloride Channel Activators
- Peripherally Acting Mu-Opioid Receptor Antagonists
- GC-C Agonists
- 5-HT4 Receptor Agonists
By Disease
- Chronic Idiopathic Constipation
- Irritable Bowel Syndrome with Constipation
- Opioid-Induced Constipation
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.