Mexico Conjunctivitis Therapeutics Market Analysis
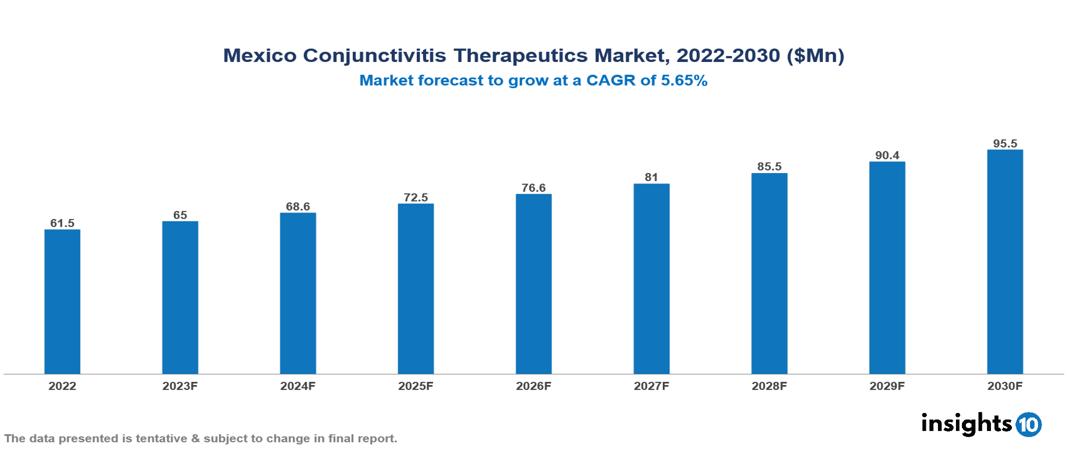
The Mexico Conjunctivitis Therapeutics Market was valued at $62 Mn in 2022 and is predicted to grow at a CAGR of 5.65% from 2023 to 2030, to $95 Mn by 2030. The key drivers of this industry include the increasing incidence of conjunctivitis, growing demand for eye care, and pharmaceutical innovation in the industry. The industry is primarily dominated by players such as Novartis, Pfizer, Bausch & Lomb, Sanofi, Santen, Allergan, and AbbVie among others.
Buy Now

Mexico Conjunctivitis Therapeutics Market Analysis Executive Summary
The Mexico Conjunctivitis Therapeutics Market is at around $62 Mn in 2022 and is projected to reach $95 Mn in 2030, exhibiting a CAGR of 5.65% during the forecast period.
Conjunctivitis, commonly known as pink eye, is characterized by the inflammation of the conjunctiva, a transparent membrane covering the surface of the eye and the inner part of the eyelid. This condition can arise from various causes, including viral or bacterial infections, allergic responses, irritation, and exposure to chemicals. Symptoms of conjunctivitis encompass redness, irritation, excessive tearing, and, in certain cases, a gritty sensation or the presence of mucous discharge. Diagnosis typically involves a comprehensive eye examination, and the appropriate treatment depends on the underlying cause. Allergic conjunctivitis may be managed with nonsteroidal anti-inflammatory medications, antihistamines, or topical steroid eye drops. Conversely, infectious conjunctivitis, particularly the bacterial variant, is commonly treated with antibiotic eye drops or ointments. Pfizer, Sanofi, Alcon, and Allergan are among the companies producing these treatment options.
Conjunctivitis has an estimated prevalence of 13% in Mexico posing a significant public health burden. The expansion of the market is driven by significant contributors, including the rising prevalence of conjunctivitis, growing demand for eye care, and pharmaceutical innovation in the industry. However, conditions such as limited access, affordability challenges, and lack of awareness in the population restrict the growth and potential of the market.
Market Dynamics
Market Growth Drivers
Surge in prevalence of conjunctivitis: Mexico City is listed among the most polluted urban areas globally, subjecting inhabitants to allergens and irritants that may induce allergic and irritant conjunctivitis. The estimated prevalence of conjunctivitis is around 13% in Mexico. This issue goes beyond major cities, affecting the overall prevalence nationwide. The increasing elderly population in Mexico is at a higher risk of chronic eye conditions such as dry eye and blepharitis, elevating their vulnerability to conjunctivitis.
Growing demand for eye care: Government initiatives in Mexico, such as Seguro Popular and Seguro Social, strive to enhance healthcare accessibility, including eye care, which may lead to a higher incidence of diagnoses and treatment for conjunctivitis. With an increase in disposable incomes, there is a greater likelihood that Mexicans will allocate resources to prioritize their health, including preventive and therapeutic eye care for conditions like conjunctivitis.
Pharmaceutical innovation: Pharmaceutical firms are currently engaged in the creation of innovative treatments for conjunctivitis, which encompass targeted therapies, combination medications, and sustained-release formulations. Acknowledging the distinct requirements of the Mexican market, these companies may work on crafting cost-effective treatment alternatives customized to the local disease patterns and demographics of patients.
Market Restraints
Affordability challenges: The cost of conjunctivitis treatments, including consultations, medications, and potential follow-up care, might be unaffordable for some segments of the population. This could limit market penetration and restrict patient adherence to treatment regimens.
Limited accessibility: Limited availability of healthcare, especially in rural regions, hinders patients' capacity to promptly diagnose and address conjunctivitis. This constraint could restrict the outreach of medical services and deter individuals from obtaining essential medication or medical care.
Lack of awareness: A lack of public knowledge regarding conjunctivitis, including its causes and available treatments, may impede early detection and promote self-medication habits. This could result in complications, missed chances for prompt intervention, and potentially restrict the market expansion for recommended treatments.
Healthcare Policies and Regulatory Landscape
The regulatory body overseeing therapeutics in Mexico is the Federal Commission for the Protection against Sanitary Risk (Comisión Federal para la Protección contra Riesgos Sanitarios or COFEPRIS). COFEPRIS is tasked with regulating and ensuring the safety, efficacy, and quality of therapeutic products, including pharmaceuticals and medical devices, in Mexico.
To secure licensure for therapeutics in Mexico, pharmaceutical companies typically must submit a comprehensive dossier to COFEPRIS. If the product meets regulatory requirements, COFEPRIS issues marketing authorization. This process is designed to guarantee that only safe and effective therapeutics enter the Mexican market, promoting public health and aligning with international regulatory standards.
For newcomers entering the therapeutic market in Mexico, the regulatory environment can be demanding, necessitating strict adherence to standards and comprehensive documentation. However, COFEPRIS aims to facilitate a transparent and efficient regulatory process. New entrants must navigate the regulatory landscape, follow COFEPRIS guidelines, and demonstrate the quality, safety, and efficacy of their therapeutic products.
Competitive Landscape
Key Players
- Novartis
- Bausch & Lomb
- Allergan
- Gilead Sciences
- Alembic Pharmaceutical
- Sanofi
- Pfizer
- AbbVie
- Santen Pharmaceuticals
- AstraZeneca
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Mexico Conjunctivitis Therapeutics Market Segmentation
By Drug Class
- Antibiotics
- Antiviral
- Antiallergic
- Others
By Treatment
- Mast Cell Stabilizers
- Decongestant
- Immunotherapy
- Antihistamines
- Non-steroidal anti-inflammatory drugs
- Olopatadine
- Epinastine
- Others
By Disease Type
- Bacterial
- Chemical
- Viral
- Allergic
By Formulation
- Ointment
- Drops
- Drugs
By End Users
- Hospitals and clinics
- Online Pharmacies
- Retail Pharmacies
- Drug Stores
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.