Mexico Congestive Heart Failure Therapeutics Market Analysis
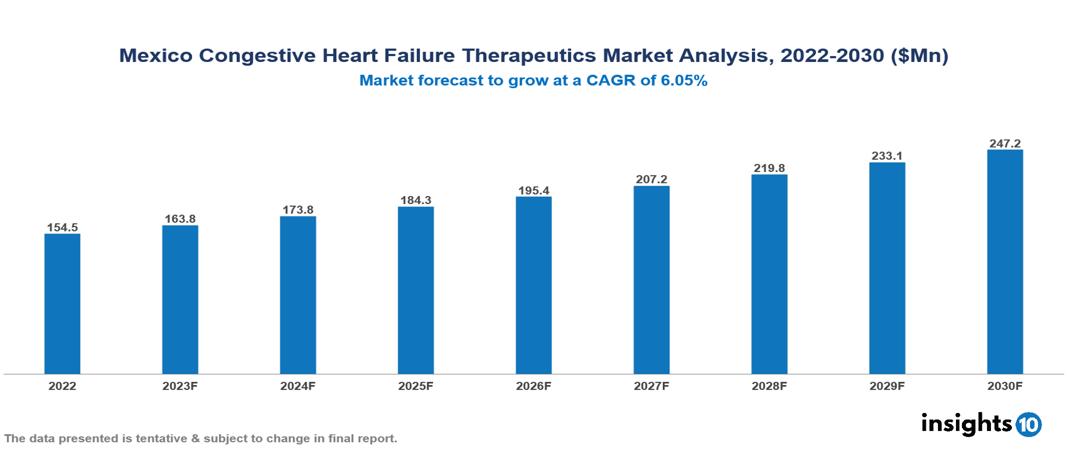
The Mexico Congestive Heart Failure Therapeutics Market is anticipated to experience a growth from $155 Mn in 2022 to $247 Mn by 2030, with a CAGR of 6.05% during the forecast period of 2022-2030. The key drivers of the market include the demographic trend of an aging population, lifestyle-related factors contributing to an increased incidence of heart failure, ongoing advancements in pharmaceutical research leading to novel therapies, and the significant impact of government initiatives. The Mexico Congestive Heart Failure Therapeutics Market encompasses various key players across different therapeutic segments, including AstraZeneca, Bayer, Bristol Myers Squibb, Novartis, Pfizer, Roche, Sanofi, PISA, Sanfer, Genomma Lab, etc, among various others.
Buy Now

Mexico Congestive Heart Failure Therapeutics Market Executive Summary
The Mexico Congestive Heart Failure Therapeutics Market is anticipated to experience a growth from $155 Mn in 2022 to $247 Mn by 2030, with a CAGR of 6.05% during the forecast period of 2022-2030.
Congestive Heart Failure (CHF) is a condition in which the heart fails to properly pump blood, resulting in inadequate circulation. Coronary artery disease, hypertension, and past incidences of myocardial infarction are all major causes of weakening heart muscle. There are two types of CHF namely systolic heart failure which impairs blood pumping, whereas diastolic heart failure hinders the heart's capacity to relax and fill with blood. Treatment options are intended to ease symptoms, improve heart function, and treat underlying concerns. ACE inhibitors, beta-blockers, and diuretics are among the most commonly given drugs. Lifestyle changes such as a low-sodium diet, frequent exercise, and weight control are important preventative measures. In extreme circumstances, procedures like implanted devices or heart transplants may be explored. Emerging techniques such as gene therapy, stem cell treatment, and precision medicine show promise for tackling the underlying causes of CHF and improving patient outcomes. Furthermore, novel medication designs targeting particular heart failure processes are entering clinical trials, raising hopes for more effective and tailored therapy choices.
Mexico has a high rate of obesity and diabetes, both of which are risk factors for CHF. Mexico has a greater frequency of CHF than other nations in the area, owing to a weak healthcare system infrastructure. Cardiovascular disorders are the primary cause of death among persons (25.5%).
The key drivers of the market include the demographic trend of an aging population, lifestyle-related factors contributing to an increased incidence of heart failure, ongoing advancements in pharmaceutical research leading to novel therapies, and the significant impact of government initiatives.
Pfizer has a significant presence in Mexico's pharmaceutical sector, which includes branded CHF drugs such as Enbrel and Xarelto. AstraZeneca is well-known for its cardiovascular portfolio, which includes proven CHF medications such as Brilinta and Forxiga. Certain domestic firms with a specialization in specific types of CHF drugs or patient groups wield great influence in that niche due to their local brand name and strong distribution networks.
Market Dynamics
Market Growth Drivers
Increasing prevalence of the disease: CHF prevalence is intimately related to demographic trends, particularly the aging population. As the population ages, the chance of getting heart failure rises, increasing the need for therapeutic measures. Furthermore, changing lifestyles characterized by sedentary activity and bad eating habits contribute to an increase in the occurrence of heart failure.
Advances in Treatment Options: The pharmaceutical industry's ongoing R&D efforts help to introduce novel and more effective CHF medicines. New drug formulations, targeted therapy, and combination treatments that improve patient outcomes and increase the overall efficacy of CHF medicines are examples of advancements.
Government Initiatives and Healthcare Policies: Government initiatives and reimbursement policies can have a substantial impact on the CHF therapeutics market. Policies that promote favorable reimbursement for specific medications or treatments can improve patient access while also encouraging pharmaceutical companies to invest in the development of new and innovative CHF remedies.
Market Restraints
Cost Constraints: The high costs associated with CHF therapeutics can be a significant barrier for many patients. Affordability issues may result in patients either being unable to access the necessary medications or facing financial strain to afford the prescribed treatments. Affordability challenges can contribute to healthcare disparities, where certain populations or socioeconomic groups may experience limited access to effective CHF therapeutics.
Regulatory Challenges: Delays in the regulatory approval process for new CHF therapeutics can impede their timely availability in the market. Extended approval timelines may postpone the introduction of innovative treatments, limiting options for patients and healthcare providers. Stringent regulatory requirements may pose challenges for pharmaceutical companies in obtaining approvals.
Limited Healthcare Infrastructure: Insufficient healthcare infrastructure, especially in certain regions with limited healthcare facilities, may create barriers for CHF patients. A lack of trained healthcare professionals, including cardiologists and specialized CHF care teams, can contribute to challenges in providing comprehensive care. Workforce shortages may limit the capacity to address the growing burden of CHF effectively.
Healthcare Policies and Regulatory Landscape
COFEPRIS, the Federal Commission for Protection against Sanitary Risks, is the primary regulatory authority in Mexico that is responsible for ensuring the safety, quality, and effectiveness of various health-related products. Operating under the Secretariat of Health, the COFEPRIS is committed to safeguarding public health by assessing and approving the registration of drugs and medical devices, conducting inspections of manufacturing facilities, and enforcing adherence to regulatory standards. COFEPRIS plays a crucial role in the healthcare system by overseeing the entire drug approval process, from the initial application to the post-market surveillance stage. The agency uses a risk-based system to classify medicinal products, with Class I being low-risk and Class III posing the highest potential danger. It also collaborates with international entities, such as the FDA, to stay informed about advancements in regulatory science and to enhance regulatory processes.
Competitive Landscape
Key Players:
- AstraZeneca
- Bayer
- Bristol Myers Squibb
- Novartis
- Pfizer
- Roche
- Sanofi
- PISA
- Sanfer
- Genomma Lab
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Mexico Congestive Heart Failure Therapeutics Market Segmentation
By Stage of Heart Failure
- Acute Heart Failure
- Chronic Heart Failure
By Drug Class
- ACE Inhibitors
- Beta Blockers
- Angiotensin 2 Receptor Blockers
- Diuretics
- Aldosterone Antagonists
- Others
By Route of Administration
- Oral
- Parenteral
By Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
By End User
- Hospitals
- Specialty Clinics
- Homecare
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.