Mexico Antibacterial (Antibiotics) Drugs Market Analysis
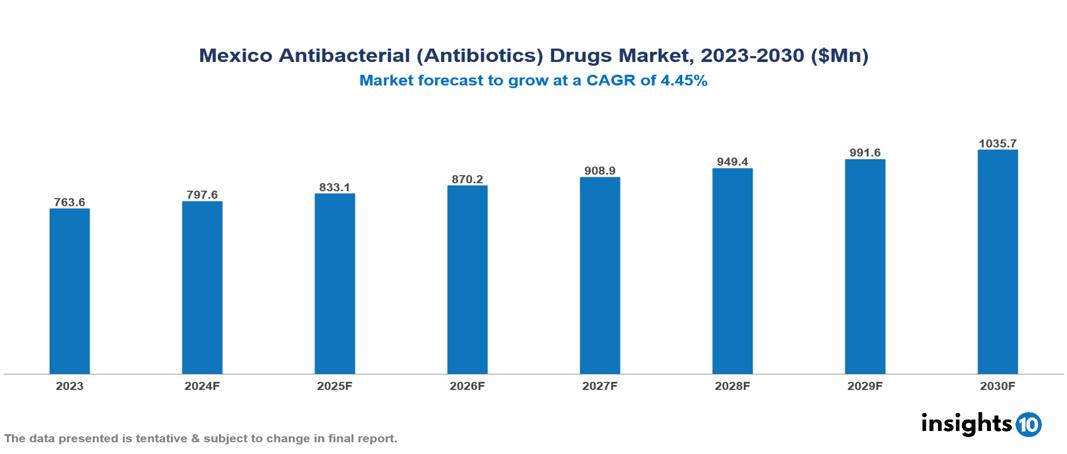
Mexico Antibacterial (Antibiotics) Drugs Market is at around $0.76 Bn in 2023 and is projected to reach $1.04 Bn in 2030, exhibiting a CAGR of 4.45% during the forecast period. The industry is expanding due to factors like medical tourism, increased resistance to antibiotics, and awareness and education. The market is dominated by key players like AbbVie (Allergan), Pfizer, AstraZeneca plc, GlaxoSmithKline plc, Johnson & Johnson, Merck & Co., Inc., Sanofi S.A., Novartis AG, Eli Lilly, and Bayer.
Buy Now

Mexico Antibacterial (Antibiotics) Drugs Market Executive Summary
Mexico Antibacterial (Antibiotics) Drugs Market is at around $0.76 Bn in 2023 and is projected to reach $1.04 Bn in 2030, exhibiting a CAGR of 4.45% during the forecast period.
Mexican medications targeted at treating bacterial infections make up the country's ever-changing antibiotic market. Taking into account the demand for antibiotics as well as the legal framework that surrounds their usage, it includes the manufacture, distribution, and consumption of antibiotics. The market's trajectory is shaped by variables including government regulations, burgeoning bacterial resistance patterns, and healthcare infrastructure, which affects healthcare outcomes and access globally.
Increasing healthcare spending and the prevalence of infectious diseases are driving the Mexican antibiotics industry. While branded alternatives are common in some markets, generic medications are still the norm. Stricter controls and a change in the market towards improved formulations are necessary due to the challenge of antibiotic resistance. The industry is anticipated to increase steadily overall due to government initiatives and the aging population. This presents profitable potential for players who prioritize compliance, affordability, and innovation.
The antibiotic industry has seen significant transformation in recent years. The market is expected to grow more and has a projected revenue of $50.91 Bn in 2023. Growing infectious diseases and innovative products are driving this growth. These improvements are the result of growing research and development, the need for new antimicrobial formulations, and the prescription of antibiotics. Urbanization and new antibiotics have an impact on market expansion.
In the Mexican antibiotic market, AbbVie (Allergan) holds a significant market share due to its range of medications, which includes Augmentin, Azithromycin, and Ciprofloxacin. Numerous bacterial infections, such as skin, urinary tract, and respiratory tract infections, are treated with these products. In 2020, AbbVie (Allergan) held a 7.4% market share and was the third-largest antibiotic supplier in Mexico.
Market Dynamics
Market Growth Drivers:
Antibiotic Resistance: Mexico encounters obstacles concerning antibiotic resistance. This fuels the need for more recent, stronger antibiotics, which spurs market innovation and results in increased sales of advanced antibiotics.
Awareness and Education: Prescription patterns and rates of usage of antibiotics are influenced by raising public and healthcare practitioner knowledge about the proper use of antibiotics.
Medical Tourism: The prevalence of medical tourism in Mexico has an impact on the use of antibiotics, especially among foreign patients looking for reasonably priced treatment. Each year 1.4 Mn to 3 Mn patients visit Mexico for medical treatment.
Market Restraints:
Regulatory Challenges: Antibiotic regulations and approval processes might hinder market entry and growth. New antibiotics may take longer and cost more to develop due to regulatory issues.
Generic Competition: Generic antibiotics, which are cheaper than branded ones, can put pressure on prices and lower profit margins for branded antibiotic manufacturers.
Economic factors: Downturns in the economy or healthcare spending can affect antibiotic demand. Financial restrictions may delay or prevent treatment, affecting antibiotic sales.
Healthcare Policies and Regulatory Landscape
The Secretariat of Health in Mexico, also known as Secretaría de Salud, is the body responsible for regulating pharmaceuticals and medical devices in addition to other aspects of health services, such as national health policy. In Mexico, the Comisión Federal para la Protección contra Riesgos Sanitarios (COFEPRIS) collaborates with the Sanitary Authorization Commission (CAS) to supervise the sanitary registration process for pharmaceuticals. The Center for Integrated Services (Centro Integral de Servicios, CIS) is in charge of requests and technical meetings for the COFEPRIS New Molecule Committee. The Mexican government rigorously controls the price of medications and places a high premium on healthcare accessibility. For expensive, innovative medications, this can be a major obstacle, making it challenging for companies to recoup their original costs.
Competitive Landscape
Key Players:
- AbbVie (Allergan)
- Pfizer
- AstraZeneca plc
- GlaxoSmithKline plc
- Johnson & Johnson
- Merck & Co., Inc.
- Sanofi S.A.
- Novartis AG
- Eli Lilly
- Bayer
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Mexico Antibacterial (Antibiotics) Drugs Market Segmentation
By Drug Class
- Cephalosporins
- Penicillin
- Fluoroquinolone
- Macrolides
- Carbapenems
- Aminoglycosides
- Sulfonamides
- 7-ACA
- Others
By Type
- Branded Antibiotics
- Generic Antibiotics
By Action Mechanism
- Cell Wall Synthesis Inhibitors
- Protein Synthesis Inhibitors
- DNA Synthesis Inhibitors
- RNA Synthesis Inhibitors
- Mycolic Acid Inhibitors
- Others
By Spectrum
- Broad-spectrum Antibiotic
- Narrow-spectrum Antibiotic
By Administration
- Oral
- Intravenous
- Others
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































