Mexico Brugada Syndrome Market Analysis
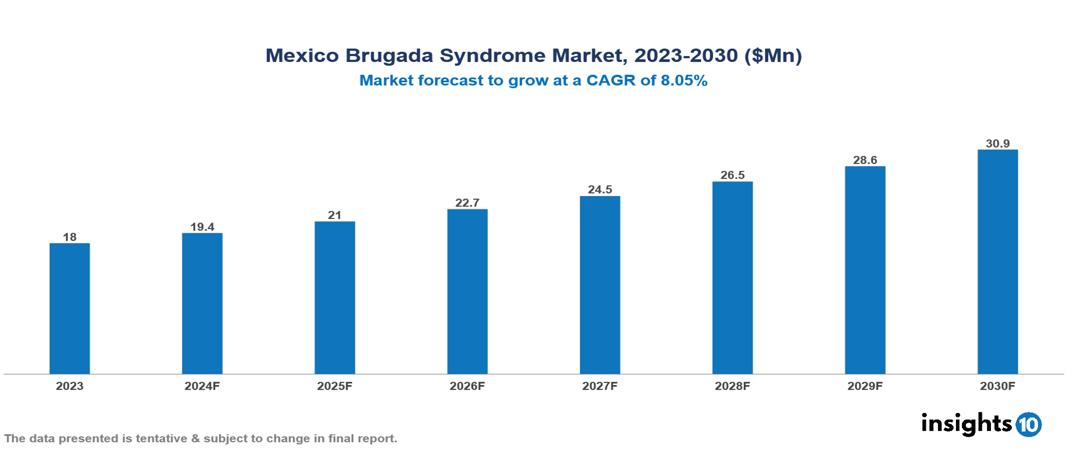
The Mexico Brugada Syndrome Market was valued at $18 Mn in 2023 and is predicted to grow at a CAGR of 8.05% from 2023 to 2030, to $30.9 Mn by 2030. The key drivers of the market include growing prevalence of cardiovascular diseases, geriatric population, and advancements in genetic testing. The prominent players of the Mexico Brugada Syndrome Market are Bayer, Roche, Teva, Medtronic, Abbott Laboratories, and Sanofi, among others.
Buy Now

Mexico Brugada Syndrome Market Executive Summary
The Mexico Brugada Syndrome Market is at around $18 Mn in 2023 and is projected to reach $30.9 Mn in 2030, exhibiting a CAGR of 8.05% during the forecast period.
Brugada syndrome is a rare, but potentially threatening, genetic condition that causes abnormal electrical activity in the heart, leading to an increased risk of sudden cardiac death. People with Brugada syndrome have an increased risk of irregular heart rhythms beginning in the lower chambers of the heart, i.e., the ventricles. Common signs and symptoms associated with Brugada Syndrome include dizziness, fainting, gasping and laboured breathing, particularly at night, irregular heartbeats or palpitations, extremely fast and chaotic heartbeat, and seizures. The risk factors for Brugada syndrome include family history of Brugada syndrome, being male, race, and fever.
Non-communicable diseases (NCDs), including CVDs, are estimated to account for 77% of total adult deaths in Mexico. The Mexico Brugada Syndrome Market is driven by significant factors such as growing prevalence of cardiovascular diseases, geriatric population, and advancements in genetic testing. However, high cost of treatment, limited awareness, and limited R&D restrict the growth and potential of the market.
The major players of the Mexico Brugada Syndrome Market are Bayer, Roche, Teva, Medtronic, Abbott Laboratories, and Sanofi, among others.
Market Dynamics
Market Growth Drivers
Growing Prevalence of Cardiovascular Diseases: CVDs account for almost a quarter of the deaths from NCDs in Mexico. The rise in cardiovascular diseases, driven by aging, lifestyle changes, and conditions like hypertension and diabetes, has led to greater attention on rare genetic disorders like Brugada syndrome. This growing focus is boosting investments in research, diagnostic tools, and advanced treatments for Brugada syndrome. As healthcare systems emphasize comprehensive cardiovascular care, the demand for effective management and therapies for this condition is increasing, contributing to market growth.
Growing Geriatric Population: In 2022, the individuals aged 65 and above in Mexico accounted for 10.61% of the total population. As individuals age, their risk of developing heart conditions, such as Brugada syndrome, increases, broadening the potential patient pool. Older adults are also more susceptible to complications from arrhythmias, underscoring the necessity for effective preventive measures and treatments. This demographic shift is likely to drive higher demand for diagnostic tools, therapeutic interventions, and monitoring devices specifically designed for the growing number of elderly patients with Brugada syndrome, thereby stimulating market growth.
Advancements in Genetic Testing: Advanced technologies facilitate the detection of specific genetic mutations associated with Brugada syndrome, allowing for earlier and more accurate diagnoses. Early detection is crucial for taking preventive measures and reducing the risk of sudden cardiac death. Furthermore, genetic testing aids in assessing risk for family members, encouraging proactive screening and management. As genetic testing becomes more accessible and affordable, it is expected to drive growth in the Brugada Syndrome Market by increasing the number of diagnosed cases and boosting demand for related treatments, devices, and monitoring services.
Market Restraints
High Cost of Treatment: The high cost of treatment and diagnostic tools for Brugada syndrome poses a major challenge to market growth. Advanced treatments, such as implantable cardioverter defibrillators (ICDs) and specialized genetic testing, are often prohibitively expensive, limiting accessibility for many patients, especially those from low- and middle-income backgrounds. This financial barrier restricts access to crucial care and treatment, hindering widespread diagnosis, early intervention, and effective management of Brugada syndrome, and thus impeding the market's overall development.
Limited Awareness: In Mexico, the limited awareness of Brugada syndrome poses a significant barrier to market growth. The general lack of understanding about the condition among the public, healthcare providers, and patients leads to delayed diagnoses, missed prevention opportunities, and inadequate management. This low level of awareness reduces the demand for diagnostic tests, treatments, and patient support services, thereby impeding the overall advancement of the Brugada syndrome market in the country.
Limited R&D: The limited research and development in Brugada syndrome significantly hinder market growth. It is rare disease which results in less investment compared to more common cardiovascular diseases, which slows progress in understanding the condition, developing new diagnostic tools, and creating innovative treatments. Consequently, medical breakthroughs are rare, leading to fewer effective therapies and diagnostic methods and ultimately restraining the market growth for Brugada syndrome.
Regulatory Landscape and Reimbursement Scenario
In Mexico, the Federal Commission for Protection against Health Risks (COFEPRIS or Comisión Federal para la Protección contra Riesgos Sanitarios) is a regulatory body of the Mexican Government; a decentralized organ of the Mexican Secretariat of Health. Among the many health-related issues it regulates in Mexico are pharmaceuticals, food safety, medical devices, organ transplants, and environmental preservation. COFEPRIS is in charge of the entire drug approval process, from the initial application to the post-market surveillance (PMS) stage, including supply and demand, advertising, commercialization, imports and exports, distribution, manufacturing, and supply.
Mexico’s pharmaceutical industry is thriving and expanding, and thus strict laws are in place to guarantee the security and effectiveness of medications made available to Mexican residents. Pharmaceutical companies seeking to sell their medications in Mexico must understand the procedure thoroughly for getting approval. The overall approval includes an extensive evaluation by COFEPRIS, accompanied by facility inspection to ensure GMP compliance. There are three different pathways for registering medicinal product with COFEPRIS. The New Drug Registration is the pathway applicable for new chemical entities that have not previously marketed anywhere in the world. Next, is the Generic Drug Registration for drugs that are similar to the already-marketed reference drugs. Lasty, Biosimilar Registration is applicable to the biological products that are similar to the approved reference biological product.
The healthcare reimbursement system in Mexico is characterized by a complex interplay between public and private sectors. For the public sector, the social security institutions are the main provider of public health insurance. Workers in the formal sector and their dependents are covered by the Instituto Mexicano del Seguro Social (IMSS). Government employees and their families are covered by the Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado (ISSSTE). To supplement public plans with extra coverage, many Mexicans opt to buy private health insurance which provides access to a greater variety of healthcare providers, such as private hospitals and specialists. The healthcare reimbursement scheme in Mexico is gradually evolving. Although many people have a safety net in public services, there are still large differences. In the future, there may be initiatives to increase public-private partnership, achieve universal coverage, and use technology to improve healthcare affordability and accessibility for all.
Competitive Landscape
Key Players
Here are some of the major key players in the Mexico Brugada Syndrome Market:
- Bayer
- Roche
- Teva
- Medtronic
- Abbott Laboratories
- Sanofi
- Boehringer Ingelheim
- Johnson & Johnson
- Siemens Healthcare
- F. Hoffmann-La Roche
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Mexico Brugada Syndrome Market Segmentation
By Diagnosis
- Electrocardiogram
- Electrophysiology (Ep) Test
- Genetic Testing
By Treatment
- Implantable Cardioverter-Defibrillator
- Drug Therapy
By End User
- Hospitals
- Clinics
- Diagnostic Centres
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































