Malaysia Oncology Therapeutics Market Analysis
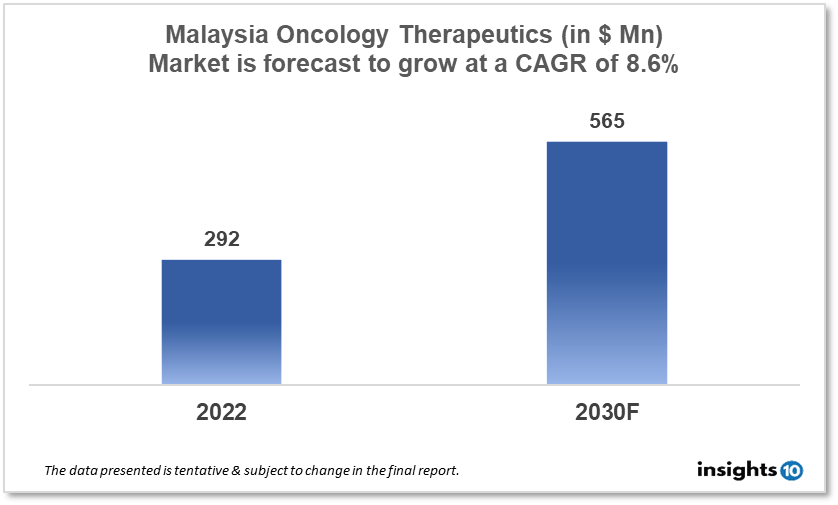
By 2030, it is anticipated that the Malaysia Oncology Therapeutics Market will reach a value of $565 Mn from $292 Mn in 2022, growing at a CAGR of 8.6% during 2022-30. The Oncology Therapeutics Market in Malaysia is dominated by a few domestic pharmaceutical companies such as Biocon Sdn Bhd, Hovid Berhad, and Pharmaniaga Berhad. The Oncology Therapeutics Market in Malaysia is segmented into different types of cancer and different therapy types. The major risk factors associated with cancer are diet, alcohol, tobacco, air pollution, and physical inactivity. The demand for Malaysia Oncology Therapeutics is increasing on account of the rise in initiatives taken by the Government of the country.
Buy Now

Malaysia Oncology Therapeutics Market Analysis Summary
By 2030, it is anticipated that the Malaysia Oncology Therapeutics Market will reach a value of $565 Mn from $292 Mn in 2022, growing at a CAGR of 8.6% during 2022-30.
Malaysia is a Southeast Asian federation of 13 states and three federal areas. It is a developing country with an upper-middle income. Cancer is one of Malaysia's worst diseases, accounting for 6,268 fatalities in 2016. Cancer is not only devastating, but it is also expensive. Cancer incidence has been rising in Malaysia in recent years, with the National Cancer Registry estimating a 3.4 % annual increase between 2007 and 2016. This has increased the demand for oncology drugs.
Malaysia's government has been investing in healthcare and attempting to enhance cancer treatment access. Malaysia's government devotes 4.1 % of its GDP to healthcare.
Market Dynamics
Market Growth Drivers Analysis
According to the Ministry of Health (MOH), 19,000 persons out of 100,000 were diagnosed with cancer in 2014, with women accounting for more than half of those affected. Biosimilars, which are lower-cost replicas of biological drugs, are gaining popularity in Malaysia. This is because original biologic drugs are expensive, and the goal is to increase cancer therapy availability. Malaysians have a high per capita income, which improves their purchasing power in the pharmaceutical and healthcare industries. The expansion of the local banking industry and the availability of FDIs attract investment in Malaysia, which enhances the country's economy. These aspects could boost Malaysia's Oncology Therapeutics Market.
Market restraints
Almost 60% of cancers in Malaysia are detected late due to the low rate of early health screening. Breast cancer is the fourth leading cause of death among Malaysian women. Although Malaysia prides itself on being a fast-growing nation with advanced health facilities, cancer patients have a comparatively high mortality rate. Medical care is becoming increasingly pricey. Malaysia's medical inflation rate was 11.5 % in 2016, with a forecasted medical inflation rate of 12.6 % in 2017. According to the Life Insurance Association of Malaysia's (LIAM) 2013 Protection Gap in Malaysia report, four to five out of every ten Malaysians do not carry life insurance. Malaysia has a strict approval process since pharmaceuticals approved in the United States or Europe typically require at least a year to be approved in Malaysia. These factors may deter new entrants into the Malaysia Oncology Therapeutics Market.
Competitive Landscape
Key Players
- Biocon Sdn Bhd: Biocon Sdn Bhd is a Malaysian subsidiary of the Indian biotechnology company Biocon Limited. It is involved in the manufacturing and marketing of biopharmaceuticals, including oncology therapeutics
- Hovid Berhad: Hovid Berhad is a Malaysian pharmaceutical company that produces a range of generic drugs and oncology therapeutics. It has a presence in several countries, including Malaysia, China, and Vietnam
- Pharmaniaga Berhad: Pharmaniaga Berhad is a Malaysian company that provides a range of pharmaceutical services, including research and development, manufacturing, and distribution. It produces a variety of drugs, including oncology therapeutics
- Kotra Pharma (M) Sdn Bhd: Kotra Pharma (M) Sdn Bhd is a Malaysian pharmaceutical company that produces a range of generic drugs, including oncology therapeutics. It has a presence in several countries, including Malaysia, Indonesia, and Vietnam
- B. Braun Medical Industries Sdn Bhd: B. Braun Medical Industries Sdn Bhd is a Malaysian subsidiary of the German medical device and pharmaceutical company B. Braun Melsungen AG. It produces a range of medical products, including oncology therapeutics
Notable Recent Deals
December 2022: The Department of Islamic Development Malaysia recognised Duopharma Biotech as the first pharmaceutical company to gain Halal certification for an oncology product (JAKIM). The Halal oncology product is manufactured at Duopharma Biotech's Highly Potent Active Pharmaceutical Ingredients (HAPI) facility in Glenmarie, Shah Alam, and is supplied to both government and private healthcare hospitals in Malaysia. It has recently been approved for export to Brunei and Singapore. Currently, the medicine is approved as an adjuvant treatment for postmenopausal women with early breast cancer and as a first-line treatment for postmenopausal women with metastatic breast cancer.
Healthcare Policies and Reimbursement Scenarios
In Malaysia, the guideline of oncology therapeutics is supervised by the National Pharmaceutical Regulatory Agency (NPRA), which is a division of the Ministry of Health. The NPRA is liable for the enlistment, observation, and control of drug items, including oncology therapeutics, to guarantee their security, adequacy, and quality. In terms of reimbursement, Malaysia has a public healthcare system called the National Health Insurance Scheme (NHIS), also known as MySalam. The NHIS provides coverage for a wide range of medical treatments, including oncology therapeutics, to eligible Malaysian citizens and permanent residents. To qualify for NHIS coverage for oncology therapeutics, patients must be diagnosed with cancer and prescribed a treatment that is approved by the NPRA.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Oncology Therapeutics Segmentation
By Application (Revenue, USD Billion):
- Blood Cancer
- ?Colorectal Cancer
- Gastrointestinal Cancer
- Gynaecologic Cancer
- Breast Cancer
- Lung Cancer
- Prostate Cancer
- ?Others
By Drugs (Revenue, USD Billion):
- Revlimid
- Avastin
- Herceptin
- Rituxan
- Opdivo
- Gleevec
- Velcade
- Imbruvica
- Ibrance
- Zytiga
- Alimta
- Xtandi
- Tarceva
- Perjeta
- Temodar
- Others
By Therapy (Revenue, USD Billion):
- Chemotherapy
- Targeted Therapy
- Immunotherapy
- Hormonal Therapy
- Others
By Route of Administration (Revenue, USD Billion):
- Oral
- Parenteral
- Others
By Distribution Channel (Revenue, USD Billion):
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- ?Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.






































