Malaysia HIV Drugs Market Analysis
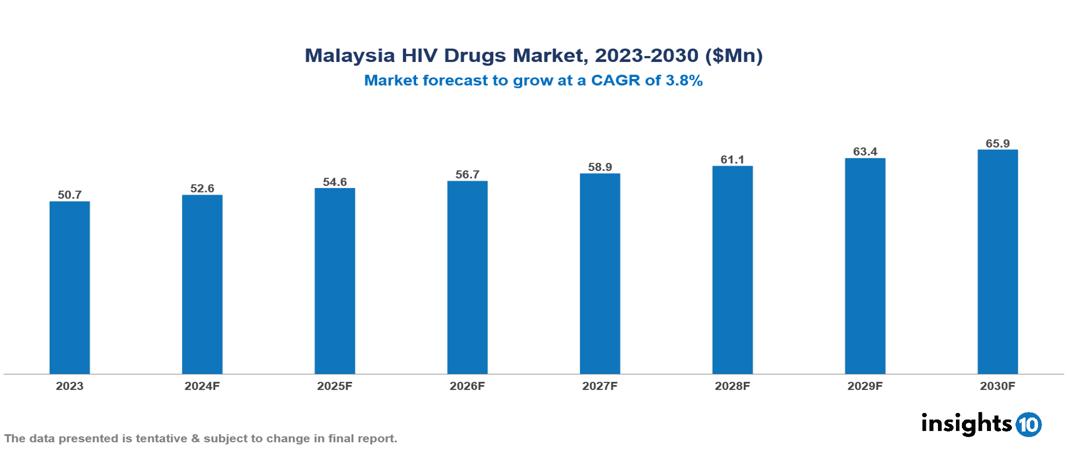
Malaysia HIV Drugs Market is at around $50.72 Mn in 2023 and is projected to reach $65.85 Mn in 2030, exhibiting a CAGR of 3.8% during the forecast period. The market is growing as a result of government initiatives, rising HIV prevalence, and the expansion of healthcare infrastructure. The market is dominated by key players like Pharmaniaga Berhad, Gilead Sciences, ViiV Healthcare, Boehringer Ingelheim International GmbH, Janssen Pharmaceuticals, Merck & Co., Bristol-Myers Squibb, AbbVie Inc., F. Hoffmann-La Roche Ltd., and Novartis.
Buy Now

Malaysia HIV Drugs Market Executive Summary
Malaysia HIV Drugs Market is at around $50.72 Mn in 2023 and is projected to reach $65.85 Mn in 2030, exhibiting a CAGR of 3.8% during the forecast period.
Malaysia's HIV Drugs Market focuses on drugs used to treat HIV/ AIDS in the nation. This market includes a range of antiretroviral medications and treatments designed to manage and slow the disease's progression. The functioning of the healthcare system, government programs, and treatment accessibility all have a significant impact on the way the market is shaped along with how the requirements of HIV patients in Malaysia are met.
The market for HIV medications in Malaysia is expanding steadily owing to government programs, rising public awareness, and better healthcare facilities. While access to newer medications and combination therapies is becoming more important, antiretroviral therapy still serves as the cornerstone of treatment. To meet the changing needs of patients and healthcare professionals, pharmaceutical companies are entering new markets and growing their market share.
HIV medication sales have increased rapidly, in 2023, the market was projected to be valued at $31.7 Bn. An increase in HIV diagnoses is the main factor fueling this growth. But as more generic substitutes become accessible and competition heats up, the market is shifting. To make therapy more accessible in developing nations, it will be necessary to address the financial obstacles to treatment, lessen HIV stigma, and provide support services.
Among the top pharmaceutical companies in Malaysia, Pharmaniaga Berhad, is a significant player in the HIV medication market in the country. The company produces and markets a variety of HIV drugs, such as antiretroviral drugs (ARVs). In addition to providing ARVs to the private sector, Pharmaniaga Berhad is a significant supplier of ARVs to the Malaysian government. The business has an excellent record of providing HIV patients in Malaysia with high-quality and reasonably priced medications.
Market Dynamics
Market Growth Drivers:
Growing HIV Prevalence: The demand for HIV medications increases as HIV/ AIDS cases become more common in Malaysia. In 2022, 86K people were living with HIV in Malaysia.
Government Funding and Initiatives: The market for HIV medications is significantly strengthened by government funding and initiatives aimed at preventing and treating HIV/ AIDS, including more funding for testing and treatment programs.
Treatment Advancements: By enhancing treatment efficacy, tolerability, and patient adherence, technological breakthroughs and innovations in HIV medication development, such as the launch of novel antiretroviral therapies (ARTs), combination therapies, and long-acting formulations increase market share.
Market Restraints:
Stigma and Discrimination: HIV/ AIDS still carries a heavy social stigma in many parts of the world, including Malaysia, despite efforts to increase awareness and lessen it. Because of this stigma, fewer people may seek out HIV medication, testing, treatment, and support services.
Cost and Affordability: Many people may find the expense of HIV medications to be prohibitive, particularly in low- and middle-income nations like Malaysia. The cost of these medications continues to be a major barrier to access for certain populations, despite efforts and programs to lower their cost.
Intellectual Property Rights and Patents: These legal protections have the potential to restrict access for individuals unable to afford name-brand medications by preventing the production of generic versions of HIV drugs, thus maintaining high costs.
Healthcare Policies and Regulatory Landscape
The Control of Pharmaceuticals and Cosmetics Regulations 1984 established the Drug Control Authority (DCA) as the executive body in charge of monitoring the registration procedure for new pharmaceuticals and biologics in Malaysia. The QUEST3 system must be used for the application process. Through QUEST's online membership registration, users can do secure online actions related to product registration applications. Complexity and possible delays may arise from the need for high-quality data and strict protocol adherence in DCA to meet international standards for Good Clinical Practice and Good Manufacturing Practice, such as those established by the International Council on Harmonization (ICH). Research on the safety and effectiveness of medications across different populations is essential in Malaysia because of the country's diverse ethnic groups. Additional clinical trials or data analysis may be necessary, which could lead to price increases and further complications.
Competitive Landscape
Key Players:
- Pharmaniaga Berhad
- Gilead Sciences
- ViiV Healthcare
- Boehringer Ingelheim International GmbH
- Janssen Pharmaceuticals
- Merck & Co.
- Bristol-Myers Squibb
- AbbVie Inc.
- F. Hoffmann-La Roche Ltd
- Novartis
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
HIV Drugs Market Segmentation
By Drug Class
- Integrase Inhibitors
- Non- Nucleoside Reverse Transcriptase Inhibitors (NRTIs)
- Combination HIV Medicines
- Others
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































