Malaysia Contraceptive Devices Market Analysis
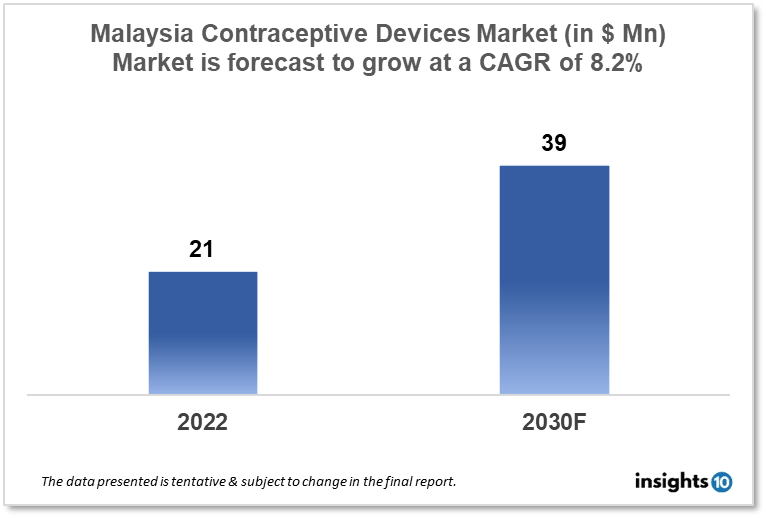
Malaysia's Contraceptive Devices Market is expected to witness growth from $21 Mn in 2022 to $39 Mn in 2030 with a CAGR of 8.20% for the forecasted year 2022-30. To encourage family planning and reproductive health, the Malaysian government has taken several actions, including subsidizing contraceptive products and services. This funding has boosted the demand for contraceptive devices. The market is segmented by type and by gender. Some key players in this market include Karex Berhad, BP Healthcare Group, Mylan Laboratories, Mankind Pharma, CooperSurgical, Pfizer, and Teva Pharmaceutical.
Buy Now

Malaysia Contraceptive Devices Healthcare Market Executive Analysis
Malaysia's Contraceptive Devices Market is expected to witness growth from $21 Mn in 2022 to $39 Mn in 2030 with a CAGR of 8.20% for the forecasted year 2022-30. Malaysia plans to utilise $16 billion on healthcare in 2023, a 7.1% increase from 2019. They predict that healthcare expenses will rise 7.6% over the following five years, reaching $22.6 billion by 2027. In Malaysia, one of the world's longest countries, 14% of the population is anticipated to be 65 or older by 2045.
In Malaysia, there were 38 maternal deaths for every 100,000 live births in 2021. In Malaysia, this translates to 38 women dying from problems connected to pregnancy for every 100,000 live births. In Malaysia, 30,906 instances of chlamydia, 25,449 cases of gonorrhoea, and 5,317 cases of syphilis were reported in 2022. When used appropriately and regularly, contraceptive methods are quite successful at preventing undesired pregnancies. This may contribute to a decrease in the number of unplanned pregnancies, which can have a major effect on people, families, and society as a whole. Contraceptive methods can assist people in spacing out their pregnancies and family planning, which can enhance mother and infant health outcomes. Additionally, using contraceptives can lower your risk of contracting STIs. With more control over their life and reproductive health thanks to contraceptive methods, women are free to pursue their educational, professional, and other ambitions without worrying about unforeseen pregnancies.
Market Dynamics
Market Growth Drivers
In order to encourage family planning and reproductive health, the Malaysian government has taken a number of actions, including subsidising contraceptive products and services. This funding has boosted demand for contraceptive products and widened access to family planning services. Malaysia, which has a population of over 30 million, has a sizable and expanding market for contraceptive devices. In addition, Malaysia's urbanisation trend is driving up the need for family planning services. Malaysia's populace is becoming increasingly accepting of and interested in contraceptive methods as a result of increased education and awareness of the value of family planning.
Market Restraints
In Malaysia, the government offers subsidies for contraceptive products and services, some customers may still find the cost of some products to be a barrier, especially those with low incomes. Contraceptive use may be stigmatised in some societies, and false information about their efficacy or negative consequences may deter some people from using them. There are various societal and cultural impediments to the use of contraceptive devices in Malaysia, despite the fact that awareness and acceptability of family planning are growing. Traditional ideas regarding family size and the use of contraception, for instance, may still be held by some people and communities.
Competitive Landscape
Key Players
- Karex Berhad (MY)
- BP Healthcare Group (MY)
- Mylan Laboratories
- Church & Dwight
- CooperSurgical
- Pfizer
- Teva Pharmaceutical
Healthcare Policies and Regulatory Landscape
In Malaysia, the Ministry of Health (MOH) is in charge of regulating contraceptive devices. To guarantee the security and effectiveness of contraceptive devices, the MOH has built a regulatory framework that consists of registration procedures, quality standards, and post-market surveillance. The organisation in charge of overseeing the registration, licencing, and post-market surveillance of medicines and medical devices in Malaysia is called the National Pharmaceutical Regulatory Agency (NPRA). Before approving the marketing of contraceptive devices and other medical items, the government assesses their efficacy, quality, and safety. The MOH's Drug Control Authority (DCA), a statutory organisation, is in charge of policing Malaysia's pharmaceutical and medical device import, export, and sales. Before approving the marketing of contraceptive devices and other medical products, the DCA assesses the products' efficacy, quality, and safety. In Malaysia, family planning policies and programmes are implemented under the direction of the National Family Planning Board (NFPB), a government organisation. To make sure that contraceptives and other family planning products are readily available and easily accessible to the general population, the agency collaborates with producers and healthcare practitioners. The MOH's Malaysian Medical Device Authority (MDA) is the regulatory body in charge of registering and overseeing medical devices in Malaysia. To assure the safety and effectiveness of medical devices, including contraceptive devices, the MDA establishes quality standards and regulatory criteria.
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Contraceptive Devices Market Segmentation
By Type (Revenue, USD Billion):
A condom is a sheath-shaped barrier device used during sexual intercourse to reduce the probability of pregnancy or a sexually transmitted infection (STI). There are both male and female condoms. With proper use, women whose partners use male condoms experience a 2% per-year pregnancy rate. Their use greatly decreases the risk of gonorrhea, chlamydia, trichomoniasis, hepatitis B, and HIV/AIDS, per the World Health Organization Updates in November 2020. To a lesser extent, they also protect against genital herpes, human papillomavirus (HPV), and syphilis. Hence, the other reasons that aid the growth of the contraception devices market are the rising occurrence of STDs and the substantial increase in the population
- Condoms
- Diaphragms
- Cervical Caps
- Sponges
- Vaginal Rings
- Intra Uterine Devices (IUD)
- Other Devices
By Gender (Revenue, USD Billion):
Based on Gender the contraceptive devices market is segmented into male and female.
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































