Malaysia Cardiac Resynchronization Therapy Market
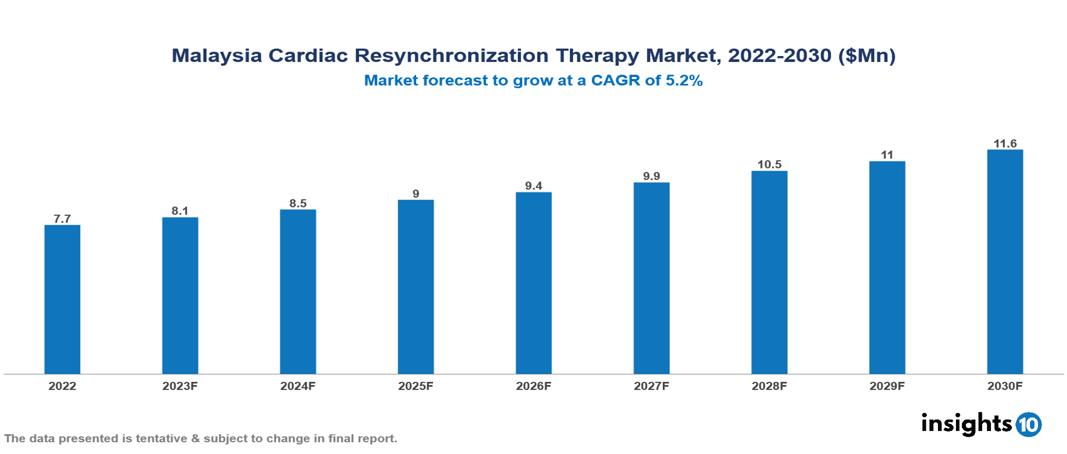
Malaysia Cardiac Resynchronization Therapy Market valued at $8 Mn in 2022, projected to reach $12 Mn by 2030 with a 5.2% CAGR. The notable increase in heart failure cases worldwide, propelled by aging demographics and lifestyle-associated cardiovascular ailments, stands as a pivotal factor fuelling the growth of the Cardiac Resynchronization Therapy (CRT) market. Presently, key players in this market encompass Abbott, Biotronik, Boston Scientific, Medtronic, Nihon Kohden, Oscor, Siemens Healthineers, Sorin Group, MicroPort CRM, and Osram Health.
Buy Now

Malaysia Cardiac Resynchronization Therapy Market Executive Summary
Malaysia Cardiac Resynchronization Therapy Market valued at $8 Mn in 2022, projected to reach $12 Mn by 2030 with a 5.2% CAGR.
Cardiac resynchronization therapy (CRT) is a medical procedure that employs a pacemaker to correct the heart's rhythm through a minimally invasive surgical approach. Positioned beneath the skin, the CRT pacemaker synchronizes the timing between the upper and lower heart chambers, ensuring coordination between the left and right sides of the heart. This treatment is particularly beneficial for individuals with heart failure, addressing inadequate pumping and fluid retention in the lungs and legs caused by the asynchronous beating of the heart's lower chambers. In cases of severe heart rhythm irregularities, CRT therapy may be supplemented by the inclusion of an implantable cardioverter-defibrillator (ICD). The CRT device connects wires from the pacemaker to both sides of the heart, utilizing biventricular pacing to ensure synchronized contractions and optimize overall heart function.
Heart disease is the leading cause of premature deaths in Malaysia, particularly affecting individuals aged 30 to 69, below the country's average life expectancy of 75. It accounted for 18.4% of medically certified deaths in this age group in 2023. Notably, heart attacks are occurring in individuals as young as their early 30s and late 20s, indicating a rising incidence among younger Malaysians. Ischemic heart disease, characterized by reduced blood flow to the heart muscle, accounts for 17.7% of all years lost to death, reflecting the severity of the issue. Poor eating habits, unhealthy lifestyle choices, and family history contribute to this concerning trend, underscoring the need for multifaceted interventions to address the growing burden of heart disease in the nation.
Medtronic's Micra AV2 and Micra VR2 leadless pacemakers, the world's smallest, have received the CE Mark. With nearly 40% longer battery life, lasting approximately 16 and 17 years respectively, they offer remote monitoring capabilities, eliminating the need for in-person clinic visits. These next-generation pacemakers introduce new algorithms to optimize atrioventricular synchrony at faster heart rates, reducing the need for in-office follow-up and serious problems. Medtronic's innovative approach is poised to reshape cardiac care, providing extended longevity, advanced programming features, streamlined monitoring for patients, and more efficient tools for healthcare professionals.
Market Dynamics
Market Growth Drivers
Increasing Incidence of CVDs: The escalating prevalence of cardiovascular diseases (CVDs) stands out as a primary catalyst propelling the expansion of the cardiac resynchronization therapy (CRT) market in Malaysia. Heart-related ailments have become a predominant cause of untimely fatalities in the country, particularly impacting individuals aged 30 to 69, falling short of the national average life expectancy of 75 years. This surge in CVD incidence underscores the heightened demand for CRT solutions in addressing and managing cardiovascular health issues in the Malaysian population.
Technological Advancements: The evolution of CRT technology is not only fuelling market growth but also catalyzing the development of innovative and high-performance products, thereby contributing to increased efficiency and enhanced user experiences in the industry.
Growing Consumer Awareness and Demand: The expansion of the CRT market in Malaysia is fuelled by the rising awareness among consumers regarding health and wellness, coupled with a growing demand for such products.
Market Restraints
High Cost of CRT Devices and Procedures: The expenses linked to CRT devices and the procedures for their implantation can pose a notable obstacle, especially in areas with constrained financial resources. Challenges related to affordability and reimbursement may restrict accessibility for a substantial segment of the population.
Infrastructure and Resource Constraints: In certain areas, the limited healthcare infrastructure and a shortage of skilled medical professionals may impede the widespread adoption of CRT. The lack of specialized facilities and well-trained personnel can restrict access to these advanced therapies.
Limited Awareness and Education: Limited awareness among healthcare professionals and the general public regarding the benefits and applications of CRT has the potential to hinder its acceptance. It might be essential to introduce educational campaigns and training programs aimed at improving awareness and understanding.
Healthcare Policies and Regulatory Landscape
The primary regulatory authority overseeing pharmaceutical treatments in Malaysia is the National Pharmaceutical Regulating Agency (NPRA), which operates under the Ministry of Health. NPRA is responsible for ensuring the quality, safety, and efficacy of pharmaceutical products through the management of processes such as medication registration, approval, and post-market surveillance. Rigorous enforcement of Good Manufacturing Practice (GMP) guidelines plays a crucial role in maintaining consistent pharmaceutical quality during production. The Pharmacy Practice and Poisons Act of 1952 governs the sale, distribution, and other pharmacy-related activities concerning therapeutic drugs. Continuous post-market surveillance is conducted to evaluate the ongoing safety and efficacy of approved medicines. The National Essential Medicines List specifies essential medications that must adhere to specific standards. Furthermore, Malaysia explores the application of Health Technology Assessment to assess the impact of emerging health technologies, including medications.
Competitive Landscape
Key Players
- Abbott
- Biotronik
- Boston Scientific
- Medtronic
- Nihon Kohden
- Oscor
- Siemens Healthineers
- Sorin Group
- MicroPort CRM
- Osram Health
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Malaysia Cardiac Resynchronization Therapy Market Segmentation
By Product
- CRT-Defibrillator
- CRT-Pacemaker
By Age
- Below 44 years
- 45-64 years
- 65-84 years
- Above 85 years
By End-Users
- Hospitals
- Cardiac care Centres
- Ambulatory Surgical Centres
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.