Malaysia Breastfeeding Accessories Market Analysis
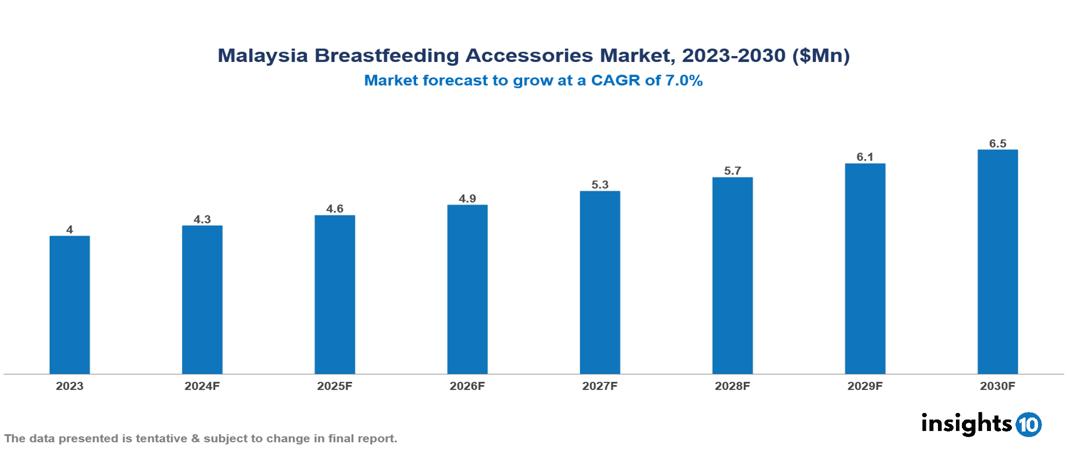
Malaysia Breastfeeding Accessories Market was valued at $4.04 Mn in 2023 and is predicted to grow at a CAGR of 7.00% from 2023 to 2030, to $6.48 Mn by 2030. The key drivers of this industry include increasing employment of women, growing awareness of breastfeeding benefits, and technological advancements in products. The industry is primarily dominated by Medala, Elvie, Willow Innovations, and Koninklijke Philips among others.
Buy Now

Malaysia Breastfeeding Accessories Market Executive Summary
Malaysia Breastfeeding Accessories Market was valued at $4.04 Mn in 2023 and is predicted to grow at a CAGR of 7.00% from 2023 to 2030, to $6.48 Mn by 2030.
Breastfeeding accessories are essential tools that aim to improve the overall breastfeeding experience for both mothers and babies, addressing various needs from comfort to convenience. Key categories include manual, electric, and hospital-grade breast pumps, which cater to different pumping needs and frequencies, while nursing pads and nipple shields provide comfort by managing milk leaks and protecting sore nipples. Breast shells help collect excess milk and shield sensitive areas, and milk storage containers ensure the safe storage of expressed milk. Nursing bras and covers offer support and discreet breastfeeding options, and breastfeeding pillows provide ergonomic support to both mother and baby during feeds. Additionally, nipple creams soothe and protect against irritation. These accessories not only enhance comfort and ease for mothers but also support health by preventing infections and maintaining milk quality, ultimately contributing to a more positive, manageable, and flexible breastfeeding experience.
In Malaysia, the prevalence of exclusive breastfeeding at six months was 48.3% among urban mothers in 2016, reflecting an improvement but still below the World Health Organization's target of 70% by 2030. Data from the National Health and Morbidity Survey (NHMS) 2022 indicates ongoing challenges, with only 40% of infants being exclusively breastfed for the first six months, which falls short of the global target of 50%.
The market is therefore driven by significant factors like increasing employment of women, growing awareness of breastfeeding benefits, and technological advancements in products. However, high cost, cultural beliefs and practices, and concerns over product safety and quality restrict the growth and potential of the market.
A prominent player in this field is Medala, in 2024, Medela AG launched a new line of breast pumps with smart technology for real-time tracking and personalized support, enhancing milk production and breastfeeding practices. Similarly, Elvie introduced an updated wearable breast pump featuring improved suction technology and longer battery life, offering greater comfort and efficiency based on user feedback. Other contributors include Willow Innovations, and Koninklijke Philips among others.
Market Dynamics
Market Growth Drivers
Increasing Employment of Women: With a female labour force participation rate of about 55% in 2023, working mothers are seeking convenient solutions to balance work and breastfeeding. Breastfeeding accessories like portable breast pumps and milk storage bags have become essential for working mothers, boosting market growth.
Growing Awareness of Breastfeeding Benefits: Awareness campaigns by healthcare professionals and organizations emphasize the health benefits of breastfeeding for both mother and child. This growing awareness encourages mothers to breastfeed longer and invest in accessories that facilitate breastfeeding, thereby driving the market.
Technological Advancements in Products: Innovative products such as electric and hands-free breast pumps have gained popularity due to their convenience and efficiency. Technological advancements in breastfeeding accessories attract consumers seeking modern solutions to enhance their breastfeeding experience, contributing to market expansion.
Market Restraints
High Cost: The cost of breastfeeding accessories can be prohibitive for many Malaysian families, with high-end electric breast pumps. This high cost limits the affordability and accessibility of these products, particularly for low-income families.
Cultural Beliefs and Practices: In some Malaysian communities, traditional beliefs may discourage the use of breastfeeding accessories, favoring direct breastfeeding. This cultural barrier can limit the market growth for accessories, as some mothers may be reluctant to adopt modern products.
Concerns Over Product Safety and Quality: Concerns about the safety and quality of breastfeeding accessories, such as reports of harmful chemicals in some products, can deter mothers from purchasing them. These safety concerns necessitate stricter regulations and quality assurance, posing challenges for manufacturers.
Regulatory Landscape and Reimbursement Scenario
In Malaysia, the regulatory framework for medical devices, including breastfeeding accessories like breast pumps, is overseen by the National Pharmaceutical Regulatory Agency (NPRA) under the Ministry of Health. The Medical Device Act 1998 provides the legal basis for regulating these products, requiring manufacturers and importers to obtain necessary registrations and licenses from the NPRA before distribution. Breastfeeding accessories are classified based on their risk profile, which affects registration requirements. Additionally, there are strict guidelines on labeling and advertising to ensure accurate consumer information.
Regarding the reimbursement scenario, Malaysia's dual healthcare system comprises public and private sectors with varying coverage for breastfeeding accessories. Public healthcare generally offers free or subsidized services, with coverage for breastfeeding accessories often limited to specific conditions or low-income groups. For instance, breast pumps may be covered in cases of premature births or lactation difficulties. In the private sector, health insurance plans vary, with some offering partial or full coverage for breastfeeding accessories, especially for mothers with particular medical needs.
Competitive Landscape
Key Players
Here are some of the major key players in the Malaysia Breastfeeding Accessories
- Medela AG
- Ameda, Inc.
- Willow Innovations, Inc.
- Koninklijke Philips N.V.
- Elvie (Chiaro Technology)
- Linco Baby
- Spectra Baby
- Hygeia Health
- NUK USA
- Mayborn Group (Tommee Tippee)
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Malaysia Breastfeeding Accessories Market Segmentation
By Product Type
- Nipple Care Products
- Breast Pumps
- Breast Shells
- Breastmilk Storage & Feeding Products
- Others
By Distribution Channel
- Online retail
- Offline retail
- Hospital pharmacies
By End User
- Hospitals
- Clinics
- Homecare Settings
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































