Malaysia Atherosclerosis Therapeutics Market Analysis
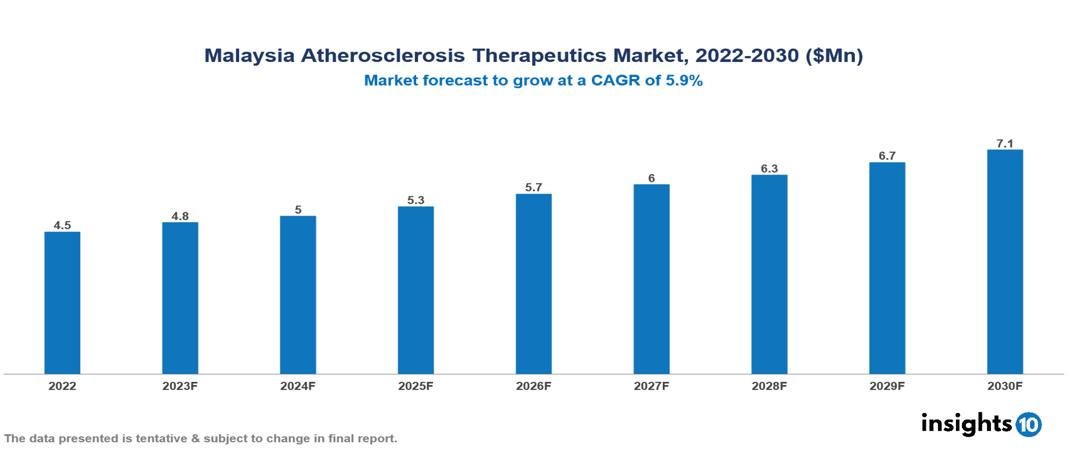
Malaysia Atherosclerosis Therapeutics Market was valued at $5 Mn in 2022 and is estimated to reach $7 Mn in 2030, exhibiting a CAGR of 5.9% during the forecast period. The demand for atherosclerosis treatments is expected to rise due to the growing cases of cardiovascular diseases, escalated by unhealthy eating habits, inactive lifestyles, and a globally aging population. The top leading pharmaceutical companies presently operating in the industry are Novartis, Pfizer, AstraZeneca, Eisai, Merck Sharp & Dohme, Sanofi, Bayer, Abbott, Johnson & Johnson and Roche
Buy Now

Malaysia Atherosclerosis Therapeutics Market Executive Summary
Malaysia Atherosclerosis Therapeutics Market was valued at $5 Mn in 2022 and is estimated to reach $7 Mn in 2030, exhibiting a CAGR of 5.9% during the forecast period.
Plaque, a sticky substance made of calcium, fat, cholesterol, and other ingredients, builds up inside the artery walls as a result of atherosclerosis. Because of this accumulation, there is a higher chance of developing severe conditions, including heart attacks and strokes, which constrict or clog blood vessels. Atherosclerosis can be caused by a variety of factors, such as high blood pressure, high cholesterol, diabetes, obesity, smoking, a family history of heart disease, inactivity, and an unhealthful diet. Several drugs, such as beta-blockers, aspirin, and statins, have shown potential in preventing or reducing the advancement of atherosclerosis.
In Malaysia, 1,869 people out of 100,000 are considered to have high-risk atherosclerotic cardiovascular disease (ASCVD); this group includes people who have a history of certain cardiovascular events as well as people who have multiple risk factors such as diabetes, hypertension, and dyslipidaemia. In individuals 18 years of age and older, the estimated prevalence of hypertension is 28.1%, while the prevalence of diabetes is 18.2% in the same age range. Dyslipidaemia, a condition marked by elevated cholesterol levels, affects about 30–40% of adult Malaysians. Atherosclerosis prevalence tends to increase with age, affecting more individuals in older age groups, and men generally exhibit a higher prevalence than women, particularly at younger ages, though the gap narrows with age, and women eventually catch up or surpass men in prevalence after menopause.
Initiation of a Phase 3 clinical trial in patients with established atherosclerotic cardiovascular disease (ASCVD) to evaluate the effect of Inclera (fenofibrate) on the risk of major adverse cardiovascular events (MACE). This important trial aims to shed light on Inclera's possible advantages and safety profile for this particular patient population. To better understand the long-term safety and efficacy of Crestor (rosuvastatin) in people with atherosclerotic disease, AstraZeneca and Eisai are working together to perform a Phase 4 trial in Malaysia. To obtain a deeper understanding of the long-term therapeutic benefits and safety issues related to Crestor use, a thorough examination is necessary.
Market Dynamics
Market Growth Drivers
Aging Population: The increasing prevalence of an aging population in Malaysia, characterized by a rising number of individuals aged 65 and above, is anticipated to nearly double from 6.4 million in 2020 to 14.7 million by 2050. This demographic shift amplifies the demand for atherosclerosis treatment drugs, as the elderly population is inherently at a heightened risk of developing atherosclerosis and related complications, thereby acting as a significant market growth driver in Malaysia.
Rising Prevalence of Risk Factors: With a substantial portion of the Malaysian population exhibiting high-risk Atherosclerotic Cardiovascular Disease and an escalating prevalence of hypertension, diabetes, and dyslipidemia driven by factors such as unhealthy lifestyles and urbanization, the expanding demand for medications to address these conditions acts as a key driver for market growth in the atherosclerosis treatment drug sector in Malaysia.
Advancements in Drug Development: To address the needs of a growing patient base and a variety of demands, pharmaceutical companies' persistent efforts to develop novel atherosclerosis drugs tailored to various pathways and patient needs are expected to accelerate market expansion in Malaysia.
Market Restraints
Affordability: A large fraction of the population has barriers to accessibility because of the high cost of modern atherosclerosis treatment drugs, as well as expenses from imported medications due to import tariffs and exchange rate changes. Even with the growing availability of generic medications, there are still gaps in affordability that mostly affect people with low incomes and those living in remote regions. This affordability issue affects a significant portion of the population that has financial difficulties and impedes the wider acceptance and accessibility of atherosclerosis drug treatment in Malaysia.
Compliance and Adherence Issues: The prolonged use of drugs in atherosclerosis treatment poses challenges such as adverse effects, forgetfulness, and low motivation for some patients. Managing numerous prescriptions for different medical conditions makes adherence problems worse, which could limit the commercial potential of atherosclerosis treatment options because of difficulties with patient compliance.
Healthcare infrastructure limitations: Inequitable access to healthcare services, particularly in remote regions, may impede prompt identification and treatment of atherosclerosis. A lack of knowledge about risk factors and financial difficulties brought on by inadequate insurance coverage for chronic conditions like atherosclerosis might cause delays. These challenges in Malaysia's healthcare infrastructure impact patient awareness, accessibility, and financial support, posing obstacles to effective atherosclerosis management.
Healthcare Policies and Regulatory Landscape
The National Pharmaceutical Regulating Agency (NPRA), which is the principal regulating body for treatment pharmaceuticals in Malaysia, operates under the Ministry of Health. The NPRA guarantees the quality, safety, and efficacy of pharmaceutical products through overseeing medication registration, approval, and post-market surveillance. To ensure constant quality in the production of pharmaceuticals, adherence to GMP guidelines is strictly enforced. The Pharmacy Practice and Poisons Act of 1952 governs the sale and distribution of therapeutic drugs, among other pharmacy-related operations. The safety and effectiveness of medicines that have been approved are evaluated by continuous post-market surveillance. Important medications that must adhere to certain standards are listed on the National Essential Medicines List. Furthermore, Malaysia investigates the application of Health Technology Assessment to assess the effects of novel health technologies, such as medications.
Competitive Landscape
Key Players
- Novartis
- Pfizer
- AstraZeneca
- Eisai
- Merck Sharp & Dohme
- Sanofi
- Bayer
- Abbott
- Johnson & Johnson
- Roche
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Malaysia Atherosclerosis Therapeutics Market Segmentation
By Therapy
- Atherosclerosis Medications
- Cholesterol-lowering Medications
- Antiplatelet drugs and Anticoagulants
- Atherosclerosis Beta Blockers
- Diuretics or Water Pills
- Angiotensin Converting Enzyme (Ace) Inhibitors
- Other Atherosclerosis Treatment Therapies
By Surgery
- Bypass Surgery (Coronary Artery Bypass Grafting (CABG))
- Angioplasty
- Atherectomy
By Drug Class
- Cholinesterase Inhibitors
- NMDA Receptor Antagonists
- Manufactured Combination
By End-Users
- Hospitals
- Specialty Clinics
- Homecare
- Others
By Distribution Channel
- Hospital pharmacies
- Clinics
- Drug stores
- Retail pharmacies
- Online pharmacies
- Other distribution channel
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.