Malaysia Actinic Keratosis Therapeutic Market Analysis
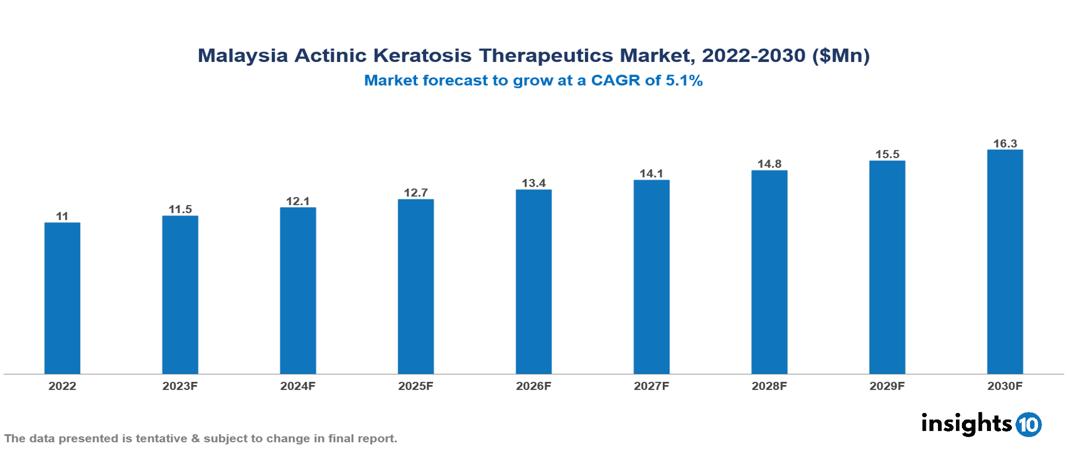
Malaysia Actinic Keratosis Therapeutic Market is expected to grow at a CAGR of 5.1% over the projected period, from $11 Mn in 2022 to about $16 Mn by 2030. The Actinic Keratosis Therapeutic Market in Malaysia is expanding steadily, propelled by many reasons such as the growing incidence of Actinic Keratosis, heightened consciousness, and enhanced accessibility owing to better healthcare facilities. Actinic Keratosis Therapeutics is being developed and marketed by a number of pharmaceutical firms, including Novartis, Amgen, Johnson & Johnson, Merck, Lumenis, and Pharmaniaga. Etc.
Buy Now

Malaysia Actinic Keratosis Therapeutic Market Executive Summary
Estimates indicate that the Japan Actinic Keratosis Therapeutic Market will reach roughly $16 Mn by 2030, up from $11 Mn in 2022, growing at a CAGR of 5.1% during the anticipated period
Actinic keratosis (AK) is a serious public health problem because it is linked to the development of squamous cell carcinoma. AK is characterized by precancerous skin lesions resulting from continuous ultraviolet (UV) exposure. While imiquimod and 5-fluorouracil as topical treatments continue to be first-choice, developments in less invasive techniques such as photodynamic therapy provide encouraging options. To maximize preventive and early detection measures in vulnerable groups, however, further research and targeted public health activities are required due to obstacles such cost accessibility and patient compliance
24.2% of ICU admissions in Malaysia have AK, underscoring the condition's notable prevalence. A prevalence of 14% in critically sick patients and 65% in sepsis patients lends more credence to this number. Older people are more vulnerable to AK because of their fragile skin and years of sun exposure. Increased sun exposure at work and lighter skin tones both increase the risk. The Malaysia Actinic Keratosis Therapeutic Market is expanding steadily, propelled by many reasons, such as the growing incidence of AK, heightened consciousness, and enhanced accessibility owing to better healthcare facilities
The key players involved in the Malaysia Actinic Keratosis Therapeutic development and marketing across the country include global giants like Novartis, Amgen, Johnson & Johnson, Merck, Lumenis, and Pharmaniaga, among others
Market Dynamics
Market Growth Drivers
Numerous reasons are propelling the Malaysia Actinic Keratosis Therapeutics Market's expansion. The high level of sun exposure in Malaysia, which is a key risk factor for AK, is one important reason. Due to its tropical climate and outdoor-loving population, Malaysia exposes its residents to high UV radiation levels, which is a major risk factor for the development of AK.
Treatment advances, including cryotherapy, laser ablation, and photodynamic therapy, provide easy and efficient solutions for patients looking for something more than just conventional creams or lotions. The availability of novel formulations with enhanced tolerance and efficacy, such as imiquimod creams and ingenol mebutate gels, expands therapeutic choices and improves patient outcomes. The future of AK therapy might be shaped by ongoing research and development into new medications and treatment modalities, which would further propel market expansion
Market Growth Restraints
Although the Malaysian AK therapy industry is growing at a promising rate, a number of obstacles prevent it from reaching its full potential. Cost and accessibility are two examples of such factors. Many patients may find some therapies, such as photodynamic therapy (PDT), to be financially unaffordable, which limits their access to treatment alternatives. Due to the concentration of dermatologists in metropolitan regions, access to specialist treatment and diagnosis is restricted for populations living in rural areas. Patients may have logistical challenges due to limited access to pharmacies that sell specialty drugs for AK in rural locations.
Delays in diagnosis and action may result from a lack of general knowledge of AK, its dangers, and available treatments. Topical drugs need to be used consistently; however, non-adherence might be a result of inconvenience or possible adverse effects, which reduces the efficacy of treatment.
Healthcare Policies and Regulatory Landscape
Malaysia is known for having one of the strongest healthcare systems in Southeast Asia. However, understanding the nuances of its healthcare policies and the regulatory landscape for therapeutics is crucial for navigating the system effectively. Skim Keselamatan Sosial Pekerjaan (SOCSO), Malaysia's national healthcare program, offers working people and their families basic healthcare coverage. It includes a variety of medical treatments, outpatient treatment, and hospitalization. The government's long-term aim for a "healthy, productive, and resilient nation" is outlined in the National Health Policy 2018–2030. It emphasizes enhancing basic healthcare, managing chronic diseases, and providing preventative care. The Ministry of Health oversees the National Pharmaceutical Regulatory Agency (NPRA), formerly known as the National Pharmaceutical Control Bureau (NPCB), which frames the Therapeutics Regulatory Landscape. It is in charge of guaranteeing the efficacy, safety, and quality of medications and medical equipment in Malaysia. For new pharmaceuticals, the NPRA has a rigorous, intricate registration procedure that includes factory inspections, quality control evaluations, and preclinical and clinical trials. The NPRA provides several regulatory avenues for the approval of pharmaceuticals, such as accelerated reviews for novel and orphan medications
Competitive Landscape
Key Players
- Merck
- Bayer
- Galderma
- Lumenis
- Sun Pharmaceuticals
- Pharmaniaga
- Duopharma Biotech
- Alphapharm
- Novartis
- GlaxoSmithKline plc.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Malaysia Actinic Keratosis Market Segmentation
By Treatment Type
- Topical Treatment
- Procedural Modality
- Photodynamic Therapy
- Others
By Drug Class
- Nucleoside Metabolic Inhibitor
- Immune Response Modifiers
- NSAIDs
- Photo enhancer
- Other Drug Classes
By Distribution Channel
- Hospital Pharmacies
- Drug Stores & Retail Pharmacies
- Online Providers
By Disease Type
- Clinical AK
- Subclinical AK
By End User
- Hospitals
- Private Dermatology Clinics
- Laser Therapy Centres
- Cancer Treatment Centres
- Spas and Rejuvenation Centres
- Homecare
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.