Kenya Hemodialysis Vascular Grafts Market Analysis
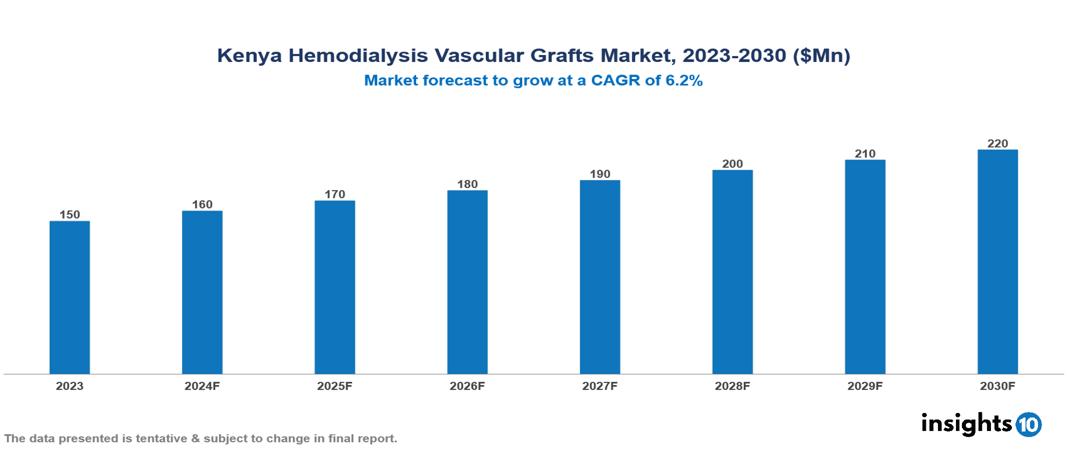
Kenya Hemodialysis Vascular Grafts Market was valued at $150 Mn in 2023 and is predicted to grow at a CAGR of 6.2% from 2023 to 2030, to $220 Mn by 2030. The key drivers of this industry include factors like the rising prevalence of chronic kidney disease, limited kidney transplant options, and growing awareness and improved diagnosis. The industry is primarily dominated by W. L. Gore, C. R. Bard, Vascudyne, and LeMaitre among others
Buy Now

Kenya Hemodialysis Vascular Grafts Market Executive Summary
Kenya Hemodialysis Vascular Grafts Market was valued at $150 Mn in 2023 and is predicted to grow at a CAGR of 6.2% from 2023 to 2030, to $220 Mn by 2030.
Hemodialysis vascular grafts are specialized devices crucial for establishing vascular access in patients, particularly those with end-stage renal disease undergoing hemodialysis. These grafts function as conduits, linking an artery to a vein to facilitate blood movement to and from the dialysis machine during treatment. They are designed to provide durable and reliable access, replacing or augmenting natural blood vessels that may not withstand the frequent punctures required in dialysis sessions. Common types include synthetic grafts made from materials like polytetrafluoroethylene (PTFE) or expanded polytetrafluoroethylene (ePTFE), and biological grafts derived from treated animal tissue. Surgical implantation involves connecting the graft in the arm or leg, ensuring proper placement and meticulous care to maintain sufficient blood flow and mitigate complications such as infection or clotting.
Globally, around 700 Mn individuals are estimated to live with chronic kidney disease (CKD). When including acute kidney injury (AKI) and individuals requiring kidney failure treatments such as dialysis or transplantation, the prevalence of kidney diseases surpasses 850 Mn people worldwide, representing more than 10% of the global population.
The market therefore is driven by significant factors like the rising prevalence of chronic kidney disease, limited kidney transplant options, and growing awareness and improved diagnosis. However, limited healthcare infrastructure, fragmented reimbursement system, and price sensitivity restrict the growth and potential of the market.
Prominent players in this field are W. L. Gore who offers the PROPATEN graft with a special heparin surface to fight clotting and the ACUSEAL graft designed for early use after implantation and C. R. Bard provides traditional ePTFE grafts and the innovative Vectra graft made from a different material that may allow for quicker access compared to standard options. Other contributors include Vascudyne, Inc., and LeMaitre among others.
Market Dynamics
Market Growth Drivers
Rising Prevalence of Chronic Kidney Disease (CKD): Kenya faces a growing burden of CKD, a major risk factor for End-Stage Renal Disease (ESRD) requiring hemodialysis. Studies suggest a prevalence of CKD ranging from 8% to 15% in the adult population. This translates to a potentially large pool of individuals at risk of progressing to ESRD.
Rising Healthcare Expenditure: Increased healthcare expenditure in Kenya is supporting better treatment options for CKD patients, including the use of advanced hemodialysis vascular grafts. This growing investment in healthcare infrastructure promotes the availability and use of high-quality medical devices.
Growing Awareness and Improved Diagnosis: Increased awareness of kidney disease and advancements in diagnostic tools could lead to earlier detection of CKD, potentially facilitating timely intervention and delaying progression to ESRD.
Market Restraints
Limited Healthcare Infrastructure: Kenya faces a shortage of healthcare facilities and trained specialists, particularly outside major urban areas. This can restrict access to hemodialysis procedures and vascular graft implantation surgery.
High Costs: The high cost of hemodialysis vascular grafts can be a significant barrier to their widespread use in Kenya. Many patients and healthcare facilities struggle with the financial burden, which can limit access to these critical devices.
Infrastructure and Training Gaps: Insufficient healthcare infrastructure and a lack of specialized training for healthcare providers can hinder the effective use of hemodialysis vascular grafts. The need for improved facilities and trained personnel affects the overall effectiveness of these devices
Regulatory Landscape and Reimbursement Scenario
In Kenya, the regulation of hemodialysis vascular grafts falls under the purview of the Pharmacy and Poisons Board (PPB), which operates under the Ministry of Health (MoH). Manufacturers aiming to register their grafts must submit a dossier with technical documentation and evidence of conformity to relevant standards, such as CE marking or a Free Sale Certificate (FSC). The PPB may also require local testing for specific Kenyan conditions. The registration process involves a thorough review of the dossier to assess the safety and efficacy of the graft, and the PPB maintains a post-market surveillance program to monitor device performance and safety.
The reimbursement landscape for hemodialysis vascular grafts in Kenya is characterized by a fragmented system, financed through a combination of public and private sources. The National Health Insurance Fund (NHIF) provides limited coverage for hemodialysis, with unclear details on vascular graft reimbursement. Private insurance plans may offer varying levels of coverage, and many patients rely on out-of-pocket payments, which poses a significant financial burden. Transparency around coverage and reimbursement rates is limited, necessitating case-by-case negotiations with hospitals and dialysis centers. Cost-effectiveness is a priority for Kenyan payers, requiring manufacturers to demonstrate the value of their grafts to secure favorable reimbursement terms.
Competitive Landscape
Key Players
Here are some of the major key players in the Kenya Hemodialysis Vascular Grafts Market
- W. L. Gore & Associates, Inc.,
- C. R. Bard, Inc.,
- Vascudyne, Inc.,
- LeMaitre,
- Getinge AB,
- Vascular Genesis,
- InnAVasc Medical, Inc.
- CryoLife, Inc.
- Merit Medical Systems
- Biovic Sdn Bhd.
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Kenya Hemodialysis Vascular Grafts Market Segmentation
Based on Raw Material
- Polytetrafluoroethylene
- Polyester
- Biological materials
- Polyurethane
Based on Indication
- Endovascular aneurysm repair
- Peripheral vascular repair
- Hemodialysis Access
Based on End-User
- Hospitals
- Clinics
- Others (Ambulatory surgical centers)
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































