Kenya Atherosclerosis Therapeutics Market Analysis
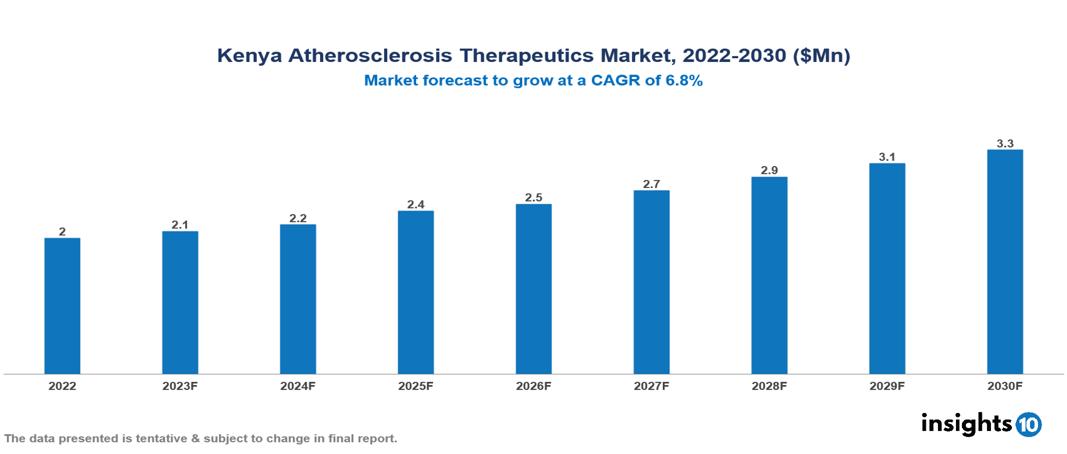
Kenya Atherosclerosis Therapeutics Market was valued at $2 Mn in 2022 and is estimated to reach $3 Mn in 2030, exhibiting a CAGR of 6.8% during the forecast period. The increasing prevalence of cardiovascular diseases, aggravated by unhealthy dietary patterns, sedentary lifestyles, and a growing aging population worldwide, is anticipated to increase the need for atherosclerosis therapeutics. Leading pharmaceutical companies currently operating in the industry are Pfizer, Novartis, AstraZeneca, Sanofi, Bayer, Abbott, Eli Lilly, GlaxoSmithKline, Teva Pharmaceutical and Roche.
Buy Now

Kenya Atherosclerosis Therapeutics Market Executive Summary
Kenya Atherosclerosis Therapeutics Market was valued at $2 Mn in 2022 and is estimated to reach $3 Mn in 2030, exhibiting a CAGR of 6.8% during the forecast period.
Atherosclerosis causes plaque, a viscous material made up of calcium, fat, cholesterol, and other substances, to build up inside the walls of arteries. This accumulation increases the chance of major illnesses, including strokes and heart attacks, which can narrow or obstruct blood vessels. Numerous variables, such as high blood pressure, high cholesterol, diabetes, obesity, smoking, a family history of heart disease, a sedentary lifestyle, and an unhealthy diet, can cause atherosclerosis. Numerous drugs, including statins, aspirin, and beta-blockers, have shown potential for slowing or stopping the development of atherosclerosis.
Kenya has high rates of risk factors for atherosclerosis; the country's population is prone to dyslipidaemia (abnormal cholesterol levels), diabetes, and hypertension, which increases the risk of atherosclerosis. Based on their lipid profile, about 30% of the population is categorized as having a moderate or medium atherogenic risk. Autopsy investigations in Kenya have revealed a noteworthy incidence of atherosclerotic lesions, with more than 25% of the participants displaying these vascular anomalies.
Verquvo, also known as inclisiran, has received approval from the US Food and Drug Administration (FDA) to reduce cholesterol in adults with familial hypercholesterolemia. The potential for this medication to be sold in Kenya is contingent upon both market and regulatory concerns. Late-stage clinical studies investigating innovative treatments, such as PCSK9 inhibitors and CETP inhibitors, that target different aspects of atherosclerosis are showing exciting success. Once these innovations are approved by regulators and prove successful in clinical trials, they may find their way into the Kenyan market. These advancements represent a changing field in the treatment of atherosclerosis and highlight the potential for novel treatments to improve cardiovascular health in Kenya.
Market Dynamics
Market Growth Drivers
Ageing population: Kenya's aging population, currently comprising 4–6% of those aged 60 and above, is anticipated to more than double to 10.3% by 2050, driving a significant demographic shift. This rise in the elderly population underscores the increasing risk of atherosclerosis due to age-related factors. With the demand for effective treatments on the rise, the growing aging demographic presents a substantial opportunity for the expansion of the atherosclerosis drug treatment market in Kenya.
Improved Diagnosis and Awareness: Increased access to diagnostic tools such as angiography and ultrasonography, supported by public-private funding and government initiatives, is promoting early detection and treatment initiation in Kenya. Greater investments in healthcare infrastructure and impactful public awareness campaigns are driving the growth of the atherosclerosis drug treatment market. The presence of advanced diagnostic technologies aids early detection, while heightened public awareness fosters demand for both preventive measures and treatments, fostering market expansion.
Advancements in Drug Development: In response to the increasing number of patients and diverse requirements, pharmaceutical companies are anticipated to intensify their ongoing endeavours in creating innovative atherosclerosis drugs, customized to different pathways and patient needs. This concerted effort is poised to expedite the expansion of the market in Kenya.
Market Restraints
High cost of drugs: The high cost of original pharmaceuticals is a major barrier to the treatment of atherosclerosis in Kenya, especially for people living in remote regions and those with minimal financial resources. Branded drugs are extremely expensive. This functions as a market constraint, impeding these demographic groups' access to necessary atherosclerosis treatments.
Lack of Awareness and Education: Lack of knowledge about atherosclerosis, its risk factors, and the treatments that are available might cause a delay in diagnosis and prevent prompt medical action. The stigma attached to long-term conditions like atherosclerosis can prevent people from getting the care they need and adhering to treatment regimens. The quality of care can also be impacted by medical professionals' lack of knowledge and experience in treating non-communicable diseases like atherosclerosis. The market for atherosclerosis treatment drugs in Kenya is severely constrained by this lack of knowledge and education, which also leads to difficulties in providing appropriate therapies, delayed diagnosis, and cultural barriers to seeking medical attention. All of these factors eventually impede market growth.
Compliance and Adherence Issues: The prolonged use of drugs in atherosclerosis treatment poses challenges such as adverse effects, forgetfulness, and low motivation for some patients. Managing numerous prescriptions for different medical conditions makes adherence problems worse, which could limit the commercial potential of atherosclerosis treatment options because of difficulties with patient compliance.
Healthcare Policies and Regulatory Landscape
In Kenya, the Ministry of Health (MOH) and the Pharmacy and Poisons Board (PPB) are principally in charge of healthcare policy and regulatory control with regard to therapeutic medications. The Ministry of Health is in charge of developing and carrying out healthcare policies and making sure that medical services, including prescription drugs, are available, affordable, and of high quality. Under the MOH, the Pharmacy and Poisons Board is responsible for regulating and supervising the safety, quality control, licensing, and registration of pharmaceutical items. Through cooperation amongst regulatory agencies, a strong framework governing the creation, authorization, and distribution of treatment medications in Kenya is established, guaranteeing adherence to safety and efficacy requirements to protect the public's health.
Competitive Landscape
Key Players
- Pfizer
- Novartis
- AstraZeneca
- Sanofi
- Bayer
- Abbott
- Eli Lilly
- GlaxoSmithKline
- Teva Pharmaceutical
- Roche
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Kenya Atherosclerosis Therapeutics Market Segmentation
By Therapy
- Atherosclerosis Medications
- Cholesterol-lowering Medications
- Antiplatelet drugs and Anticoagulants
- Atherosclerosis Beta Blockers
- Diuretics or Water Pills
- Angiotensin Converting Enzyme (Ace) Inhibitors
- Other Atherosclerosis Treatment Therapies
By Surgery
- Bypass Surgery (Coronary Artery Bypass Grafting (CABG))
- Angioplasty
- Atherectomy
By Drug Class
- Cholinesterase Inhibitors
- NMDA Receptor Antagonists
- Manufactured Combination
By End-Users
- Hospitals
- Specialty Clinics
- Homecare
- Others
By Distribution Channel
- Hospital pharmacies
- Clinics
- Drug stores
- Retail pharmacies
- Online pharmacies
- Other distribution channel
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.