Kenya Allergy Therapeutics Market Analysis
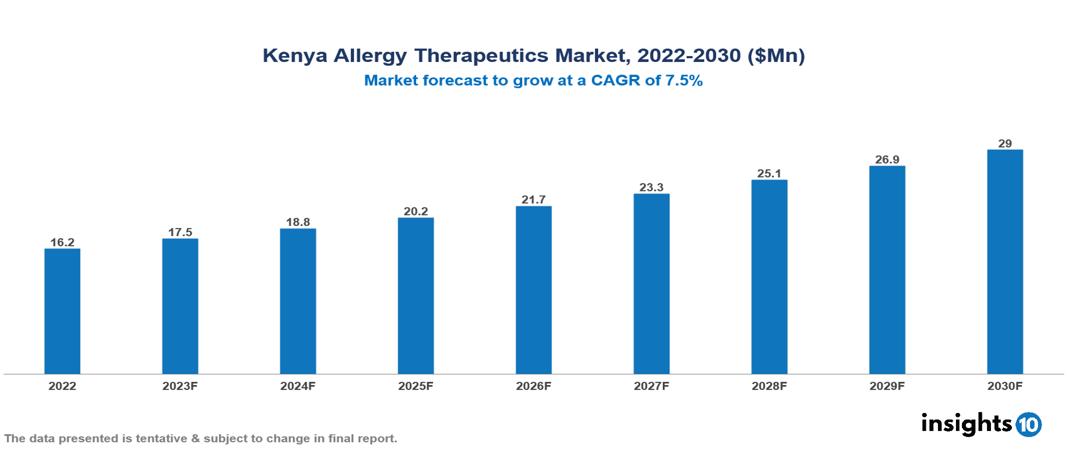
Kenya Allergy Therapeutics Market was valued at $16 Mn in 2022 and is estimated to reach $29 Mn in 2030, exhibiting a CAGR of 7.5% during the forecast period. The growing numbers of allergic disorders and associated health problems is driving up the need for allergy treatment drugs. GlaxoSmithKline, Novartis, Sanofi, AstraZeneca, Bayer, Pfizer, Abbott, Pharmanet Kenya Ltd., Mepharm Kenya Ltd. and Mediheal Kenya Ltd. are top leading pharmaceutical companies that are currently operating in the market.
Buy Now

Kenya Allergy Therapeutics Market Executive Summary
Kenya Allergy Therapeutics Market was valued at $16 Mn in 2022 and is estimated to reach $29 Mn in 2030, exhibiting a CAGR of 7.5% during the forecast period.
Allergens are substances found in the environment that can occasionally cause allergic reactions in the body. Common allergies comprise items such as dust mites, pollen, and mildew, as well as specific foods like milk, eggs, soy, and almonds. Allergies can cause a wide range of symptoms, from minor ones like a runny nose and itchy eyes to serious ones like anaphylaxis, which is marked by unconsciousness and breathing problems. Medications such as antihistamines and nasal corticosteroids can be prescribed, while immunotherapy and allergen avoidance techniques are viable forms of treatment.
An estimated 13.8% of school-age children in Kenya are expected to have bronchial asthma, which is a rather high percentage when compared to certain other nations and possibly lower than rates seen in Western nations. Specifically, in this case, allergens such as peanuts and cow's milk may be more common causes of asthma. Furthermore, skin allergies, specifically atopic dermatitis or eczema, are very common in Kenya, especially in young people. These allergy patterns are caused by a variety of variables, including environmental factors like pollen, dust mites, and air pollution. A family history of allergies might increase the risk, so genetics also play a part. Research on how dietary and lifestyle factors may affect the prevalence of allergies is still underway. Moreover, geographical disparities within Kenya, resulting from changes in temperature, pollution levels, and dietary practices, could also be a factor in the wide range of allergy prevalence rates observed throughout the nation. Recently, Mepharm Kenya Ltd. announced plans to collaborate with a research institute to develop a new antihistamine medicine that will be produced domestically. The company highlighted the medication's potential affordability benefits, specifically for the Kenyan market. This program demonstrates Mepharm's constant commitment to research and development in line with regional healthcare needs.
Pharmanet Kenya Ltd. made a big move by expanding its capacity to produce nasal corticosteroids and generic antihistamines, with the main objective being to improve the availability and cost-effectiveness of allergy drugs in Kenya.
Market Dynamics
Market Growth Drivers
Rising Incidence of Allergies: Allergies impact a huge percentage of the population, especially those with respiratory diseases like asthma and allergic rhinitis. These diseases may be becoming more common due to factors including dust mites, changing dietary patterns, and environmental pollutants.
Shifting Treatment Trends: The increasing need for personalized medical plans that take into account the unique demands and triggers of each patient may open up new markets for tailored allergy treatment alternatives. The market is growing as a result of the growing use of telehealth platforms, which facilitate better access to physicians and prescription medications.
Urbanization and Changing Lifestyles: The trend toward an increase in the need for allergy treatment pharmaceuticals is mostly being driven by urbanization and changes in lifestyle, such as greater exposure to indoor allergens such dust mites and outdoor pollutants.
Market Restraints
High Cost of Treatment: Treatments for allergies can be quite expensive for certain patients, particularly if they involve Allergen Immunotherapy methods such as immunotherapy injections and SLIT tablets. Allergy drugs high cost may prohibit people from using and accessing them broadly, which would restrict their market penetration.
Regulatory Challenges: Navigating the regulatory landscape for the importation and registration of innovative allergy medicines is a challenging and time-consuming task for pharmaceutical companies. This complexity can prevent the market from growing as it impedes the timely introduction of fresh therapeutic alternatives.
Treatment Compliance and Adherence: In order for allergy drugs, particularly immunotherapy therapies, to work, patients need to follow long-term treatment regimens. Barriers such as exorbitant costs, intricacy, or unsatisfactory results leading to inadequate patient compliance could diminish the efficacy of therapy and hinder the market's expansion.
Healthcare Policies and Regulatory Landscape
The Pharmacy and Poisons Board (PPB), which functions under the parameters of the Pharmacy and Poisons Act, is responsible for the regulatory oversight and development of drug-related healthcare policy in Kenya. The PPB is in charge of guaranteeing the effectiveness, safety, and calibre of medicines, particularly those used in medical treatment. The board is in charge of several facets of the pharmaceutical industry, such as licensing pharmaceutical professionals, registering products, and controlling the import, distribution, and sale of medications. Another essential step in the approval process for pharmaceutical products is adherence to good manufacturing practices. By enforcing the norms and laws that control the pharmaceutical business in Kenya, the PPB plays an essential role in protecting public health.
Competitive Landscape
Key Players
- GlaxoSmithKline
- Novartis
- Sanofi
- AstraZeneca
- Bayer
- Pfizer
- Abbott
- Pharmanet Kenya Ltd.
- Mepharm Kenya Ltd.
- Mediheal Kenya Ltd.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Kenya Allergy Therapeutics Market Segmentation
By Treatment Type
- Anti-allergy drugs
- Immunotherapy
By Type of Allergy
- Eye allergy
- Asthma
- Skin allergy
- Food allergies
- Rhinitis
- Other allergy types
By Route of Administration
- Oral
- Inhalers
- Intranasal
- Other routes of administration
By Distribution Channel
- Hospital pharmacies
- Retail pharmacies
- Online pharmacies
- Other distribution channel
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.