Japan Patient Engagement Solutions Market Analysis
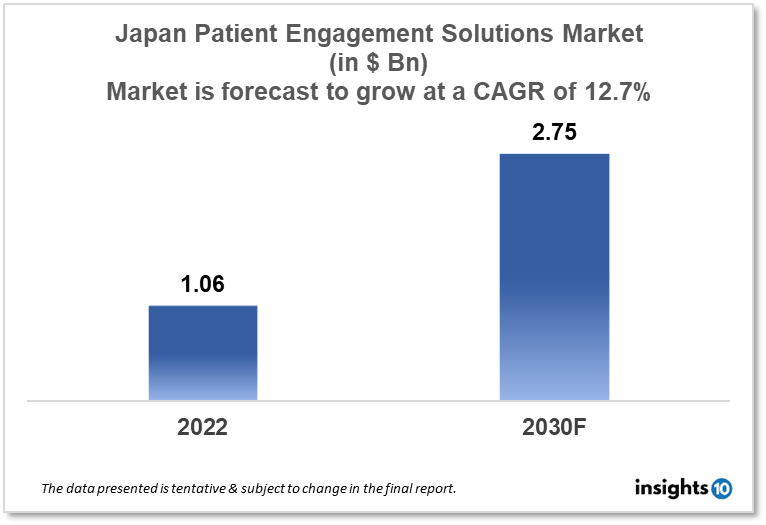
Japan's patient engagement solutions market is projected to grow from $1.06 Bn in 2022 to $2.75 Bn by 2030, registering a CAGR of 12.7% during the forecast period of 2022-30. The main factors driving the growth would be technological advancements, increasing healthcare spending, the aging population, government support, and changing patient preferences. The market is segmented by component, delivery mode, application, therapeutic area, functionality, and end-user. Some of the major players include CureApp, Takeda Pharmaceutical, NTT Data, M3 and Oracle.
Buy Now

Japan Patient Engagement Solutions Market Executive Summary
Japan's patient engagement solutions market is projected to grow from $1.06 Bn in 2022 to $2.75 Bn by 2030, registering a CAGR of 12.7% during the forecast period of 2022-30. Healthcare spending in Japan increased from $4,256 to $4,360 per person in 2018, although it decreased from 10.75% to 10.74% of GDP in 2019. In comparison to other developed nations worldwide, Japan spends more on healthcare.
A patient engagement solution is a cloud-based platform that enables healthcare providers to enhance the patient experience. In-depth patient engagement is achieved by sophisticated patient engagement software at every stage of the patient journey, from booking to triage to follow-up.
Over the past few years, Japan's Patient Engagement Solutions have advanced steadily. As a result of DIA's efforts to promote these dialogues in the market, drug companies and patients who had not previously appreciated their value have had more opportunities to discuss clinical trials.
Pharmaceutical companies have been attempting to incorporate informed consent forms into their databases in order to boost patient understanding of their protocols. The promotion of these activities must nevertheless overcome a number of obstacles in the meantime.
Market Dynamics
Market Growth Drivers
The Japanese patient engagement solutions market is expected to be driven by factors such as technological advancements, increasing healthcare spending, an ageing population and government support. Moreover, patients in Japan are becoming more accustomed to receiving care that is patient-centred and prioritises their needs and preferences which is further driving the growth of these solutions in the country.
Market Restraints
The burden of chronic diseases and Japan's ageing population are driving up healthcare expenditures despite the country's high per capita spending on healthcare. The adoption and scalability of patient engagement solutions will be hindered by a lack of healthcare funding, especially in areas with limited resources.
Competitive Landscape
Key Players
- CureApp (JPN)
- Takeda Pharmaceutical (JPN)
- NTT Data (JPN)
- M3 (JPN)
- Oracle
Notable Deals
August 2022: CureApp has announced that it has raised $51.5 Mn in a series G financing from global investment company Carlyle. CureApp plans to boost its marketing and product development platform globally and extend its sales and distribution network by utilising the investment firm's knowledge of the international healthcare market.
Healthcare Policies and Regulatory Landscape
The government in Japan has implemented a variety of regulations to raise the standard of care and encourage the use of digital healthcare technologies, such as patient interaction tools. A new regulatory framework for digital health technology, such as patient interaction tools, was introduced by the Japanese government in 2018. The new framework aims to promote the creation and uptake of ground-breaking digital health solutions while protecting patient privacy and safety.
The Ministry of Health, Labour, and Welfare is responsible for regulatory control of patient engagement solutions under the new framework (MHLW). The Pharmaceuticals and Medical Devices Agency (PMDA) must approve solutions that are categorised as medical devices before they may be commercialised in Japan, including remote monitoring devices and diagnostic instruments.
Reimbursement Scenario
The Ministry of Health, Labour, and Welfare oversees the government's establishment of reimbursement guidelines for patient engagement solutions in Japan (MHLW). Solutions for patient involvement that have received the Pharmaceuticals and Medical Devices Agency's (PMDA) stamp of approval can be eligible for payment through the federal health insurance program.
Depending on the type of solution and the particular use case, the reimbursement rate for patient engagement solutions may differ in Japan due to the complexity of the country's reimbursement system. Patient engagement solutions that are deemed medically necessary and offer patients clinical benefits are generally more likely to be covered by insurance.
1. Executive Summary
1.1 Service Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Healthcare Services Market in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Services
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Patient Engagement Solutions Market Segmentation
By component (Revenue, USD Billion):
- Hardware
- Software
- Standalone Software
- Integrated Software
- Services
By Delivery Mode (Revenue, USD Billion):
- PREMISE Mode
- CLOUD-BASED Mode
By Application (Revenue, USD Billion):
- Health Management
- Home Health Management
- Social
- Financial Health Management
By Therapeutic area (Revenue, USD Billion):
- Chronic Diseases
- Cardiovascular Diseases (CVD)
- Diabetes
- Obesity
- Other chronic Diseases
- Women's Health
- Fitness
- Other Therapeutic Areas
By Functionality (Revenue, USD Billion):
- E-Prescribing
- Virtual Consultation
- Patient/Client Scheduling
- Document Management
By End-user (Revenue, USD Billion):
- Providers
- Payers
- Patients
- Other End users
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































