Japan Oculopharyngeal Muscular Dystrophy (OPMD) Market Analysis
Japan Oculopharyngeal Muscular Dystrophy (OPMD) market is projected to grow from $xx Mn in 2023 to $xx Mn by 2030, registering a CAGR of xx% during the forecast period of 2023 - 2030. The market for Oculopharyngeal Muscular Dystrophy (OPMD)is growing due to increase spending in research and development activities by pharmaceutical companies, incentives provided by regulatory authorities like orphan drug designations and accelerated approval pathways, patient support and advocacy programmes which help to increase awareness about disease and provide support to patients. Bristol-Myers Squibb, BioMarin, Sarepta Therapeutics, Benitec Biopharma Inc., Bioblast Pharma, PTC Therapeutics, NS Pharma, Nobelpharma Co., Ltd ,Santhera Pharmaceuticals, Pfizer Inc. ,Marathon Pharmaceuticals, Fibrogen, GSK are the key global market players.
Buy Now

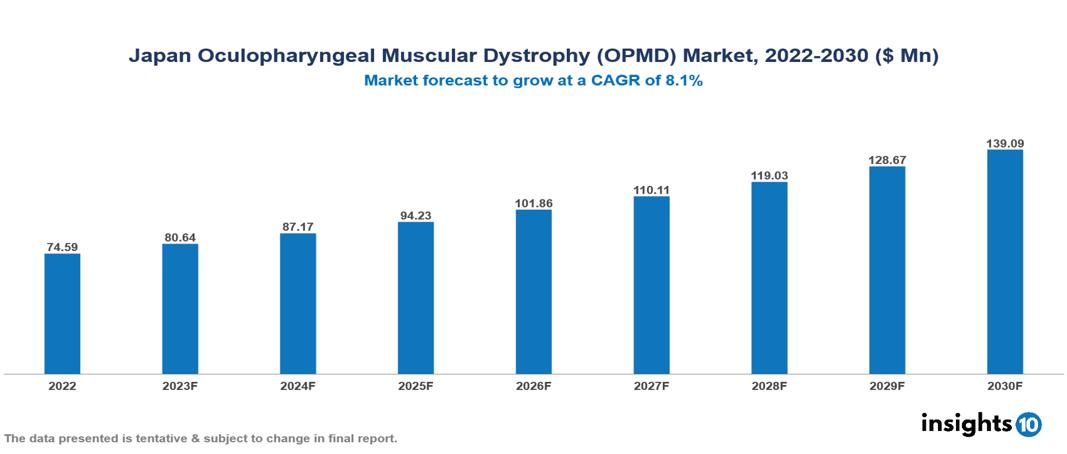
Japan Oculopharyngeal Muscular Dystrophy (OPMD) Market Analysis Summary
Japan Oculopharyngeal Muscular Dystrophy (OPMD) Market is valued at around $74.59 Mn in 2022 and is projected to reach $139.09 Mn by 2030, exhibiting a CAGR of 8.1% during the forecast period 2023-2030.
An uncommon hereditary condition called oculopharyngeal muscular dystrophy (OPMD) damages the muscles of the eyes and throat. The quality of life of the patient may be impacted by the progressive nature of this condition, which frequently causes swallowing issues and eyelid drooping. Since OPMD currently has no known cure, treatment focuses on symptom management and improving the quality of life of affected individuals. To address swallowing and speech issues, treatment methods may include speech therapy and swallowing exercises. In some situations, surgical procedures to relieve symptoms, such as cricopharyngeal myotomy or eyelid surgery, may be considered. Gene therapies, small molecule drugs, and other novel treatment modalities aimed at targeting the underlying genetic mutations or disease mechanisms are also being developed. BB-301 (Pabparna) is under development for the treatment of dysphagia associated with oculopharyngeal muscular dystrophy, a type of congenital myopathy. The therapeutic candidate is administered through an intramuscular route. The therapeutic candidate is developed based on DNA-directed RNA interference (RNAi) technology that silences unwanted or disease-causing genes. Bristol-Myers Squibb, BioMarin, Sarepta Therapeutics, Benitec Biopharma Inc., Bioblast Pharma, PTC Therapeutics, NS Pharma, Nobelpharma Co., Ltd, Santhera Pharmaceuticals, Pfizer Inc., Marathon Pharmaceuticals, Fibrinogen, GSK are the key global market players.
Market Dynamics
Market Drivers
Research and development activities by pharmaceutical companies, incentives provided by regulatory authorities like orphan drug designations and accelerated approval pathways, patient support and advocacy programmes which help to increase awareness about the disease and provide support to patients all act as market growth drivers.
Market Restraints
Limited patient populations, difficulties in diagnosis and high costs associated with research and development all act as market growth restraints.
Key players
uniQure Ionis Pharmaceuticals Alnylam Pharmaceuticals Catalyst Biosciences Pfizer Sanofi Shire plc BioMarin Pharmaceutical Inc. Orchard Therapeutics plc PTC Therapeutics Inc.1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Market Segmentations For Japan Oculopharyngeal Muscular Dystrophy (OPMD) market
By Treatment
- Symptomatic treatment
- Disease-modifying therapies
By Distribution Channels:
- Hospital pharmacies
- Retail pharmacies
- Online pharmacies
- Others
By end users
- Hospitals
- Diagnostic centres
- Clinics
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































