Japan Lipid Disorder Therapeutics Market Analysis
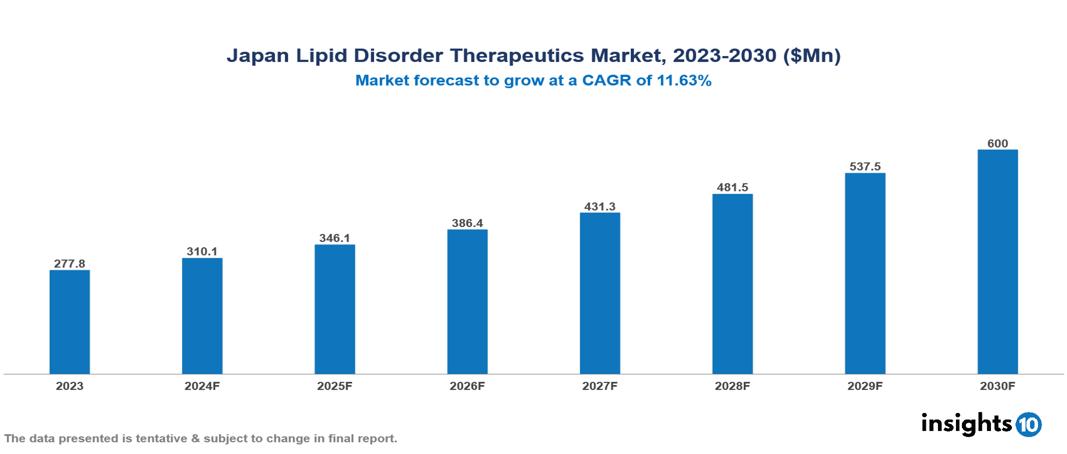
The Japan Lipid Disorder Therapeutics Market was valued at $277.8 Mn in 2023 and is predicted to grow at a CAGR of 11.63% from 2023 to 2030, to $600 Mn by 2030. Japan Lipid Disorder Therapeutics Market is growing due to the Rising Prevalence of Cardiovascular Disease, Growing Awareness, and Government Initiatives, and Technological Advancements. The industry is primarily dominated by players such as Sanofi, Sun Pharmaceutical Industries Ltd.Pfizer, Inc., GlaxoSmithKline plc, Novartis AG, Merck & Co., Inc., Amgen Inc., Takeda Pharmaceutical Company Limited, AbbVie Inc., Viatris, AstraZeneca PLC, and Dr. Reddys Laboratories Ltd.
Buy Now

Japan Lipid Disorder Therapeutics Market Executive Summary
Japan Lipid Disorder Therapeutics Market is at around $277.8 Mn in 2023 and is projected to reach $600 Mn in 2030, exhibiting a CAGR of 11.63% during the forecast period.
A lipid disorder is defined as an excess of fats or lipids in the bloodstream. Elevated triglyceride or cholesterol levels can increase the risk of heart disease and stroke. At first, a patient may not show any symptoms; nevertheless, over time, they may manifest as chest pain, yellowish deposits on the skin (xanthomas), or fatty deposits around the eyes (xanthelasmas). A healthy diet, consistent exercise, and quitting smoking are a few examples of lifestyle changes that are commonly included in treatment. Statins or fibrates are two more drugs that may be suggested to help lower cholesterol levels. It is imperative to consult a healthcare expert for an appropriate diagnosis and treatment plan.
In Japan, the prevalence of lipid disorders, including hyperlipidemia, is influenced by demographic factors such as age and gender, with higher rates observed in older adults. The prevalence of dyslipidemia is estimated at 56.6% for males and 47.2% for females. Healthcare expenses for lipid disorder therapeutics are significant due to Japan's aging population and the associated increase in chronic diseases. Efforts to manage these conditions involve lifestyle interventions and pharmacotherapy, highlighting the burden on the healthcare system. The market therefore is driven by significant factors like rising prevalence of cardiovascular disease, growing awareness and government initiatives, and technological advancements. However, the strict regulatory landscape, patent expiry, and generic erosion, cost-effectiveness concerns restrict the growth and potential of the market.
Sun Pharma launches a first-in-class oral drug, Bempedoic Acid for reducing LDL cholesterol under the brand name Brillo.
Market Dynamics
Market Growth Drivers
Rising Prevalence of Cardiovascular Disease: An aging population and unhealthy lifestyles in Japan are leading to a significant increase in cardiovascular diseases (CVDs). Dyslipidemia, a type of lipid disorder, is a major risk factor for CVDs like atherosclerosis, heart attacks, and strokes. In fact, nearly 30% of the Japanese population suffers from dyslipidemia, highlighting the severity of the issue. This growing disease burden creates a strong demand for effective lipid-lowering therapies.
Growing Awareness and Government Initiatives: Public health awareness campaigns and government initiatives aimed at promoting early diagnosis and treatment of lipid disorders are positively impacting the market. Increased awareness encourages individuals to get screened and seek treatment, driving the market growth.
Technological Advancements: Pharmaceutical companies are constantly developing new and improved lipid-lowering medications. This includes new drug classes with better efficacy and fewer side effects. Advancements in diagnostic tools for lipid disorders further contribute to market growth.
Market Restraints
Strict Regulatory Landscape: Japan rigorous drug approval process, known for its meticulousness and focus on safety, can significantly delay the introduction of novel and potentially life-saving therapies. This lag can prevent patients from accessing the latest advancements in lipid-lowering medications, hindering market progress and potentially impacting patient outcomes.
Patent Expiry and Generic Erosion: As patents on established and commercially successful lipid-lowering drugs expire, the market opens up for generic alternatives. While generics offer a significant cost advantage to patients and healthcare systems, they can erode market share and revenue for branded drugs. This price pressure can stifle investments in research and development for the next generation of therapies, potentially slowing down innovation in the long run.
Cost-Effectiveness Concerns: Balancing the high cost of cutting-edge lipid-lowering drugs with budgetary constraints in healthcare is a constant challenge. Stringent reimbursement policies and a focus on cost-effectiveness analyses determine which treatments receive insurance coverage. This can limit patient access to some potentially life-changing therapies, particularly those catering to niche patient groups or offering incremental benefits over existing options. For instance, a study found that only 30% of patients in need of advanced lipid-lowering drugs received coverage under stringent reimbursement criteria, highlighting the gap in access to essential treatments.
Regulatory Landscape and Reimbursement Scenario
Japan enforces a rigorous healthcare regulatory framework overseen by the Ministry of Health, Labour and Welfare (MHLW). This powerful ministry dictates national health policies, licenses medical professionals and facilities, and plays a critical role in ensuring the safety and effectiveness of medications and medical devices. The MHLW collaborates with the Pharmaceuticals and Medical Devices Agency (PMDA), a specialized agency responsible for meticulous drug trials, approvals, and post-market surveillance. This dual focus on policy and technical expertise fosters a comprehensive regulatory system.
Japan's healthcare reimbursement system is cost-conscious. The Ministry of Health, Labour, and Welfare (MHLW) reviews reimbursement fees for drugs and services every two years, often aiming for reductions. This approach aims to control public health spending but can limit healthcare providers' profit margins and potentially stifle innovation in treatments with higher costs. Scenario discussions often revolve around balancing cost control with ensuring access to necessary treatments. Some proposals suggest increasing out-of-pocket spending for patients to offset costs, while others advocate for more streamlined cost-effectiveness assessments to ensure valuable new treatments are adequately reimbursed.
Competitive Landscape
Key Players
Here are some of the major key players in the Japan Lipid Disorder Therapeutics Market:
- Sanofi
- Sun Pharmaceutical Industries Ltd.
- Pfizer, Inc.
- GlaxoSmithKline plc
- Novartis AG
- Merck & Co., Inc.
- Amgen Inc.
- Takeda Pharmaceutical Company Limited
- AbbVie, Inc.
- Viatris
- AstraZeneca PLC
- Dr. Reddy’s Laboratories Ltd.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Japan Lipid Disorder Therapeutics Market Segmentation
By Drug Type
- Atorvastatin
- Fluvastatin
- Simvastatin
- Pravastatin
- Others
By Indication
- Hypercholesterolemia
- Dysbetalipoproteinemia
- Familial Combined Hyperlipidemia
- Others
By Product type
- Statins
- PCSK9 Inhibitors
- Nicotinic Acid
- Bile Acid Sequestrants
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































