Japan Exosome Research Market Analysis
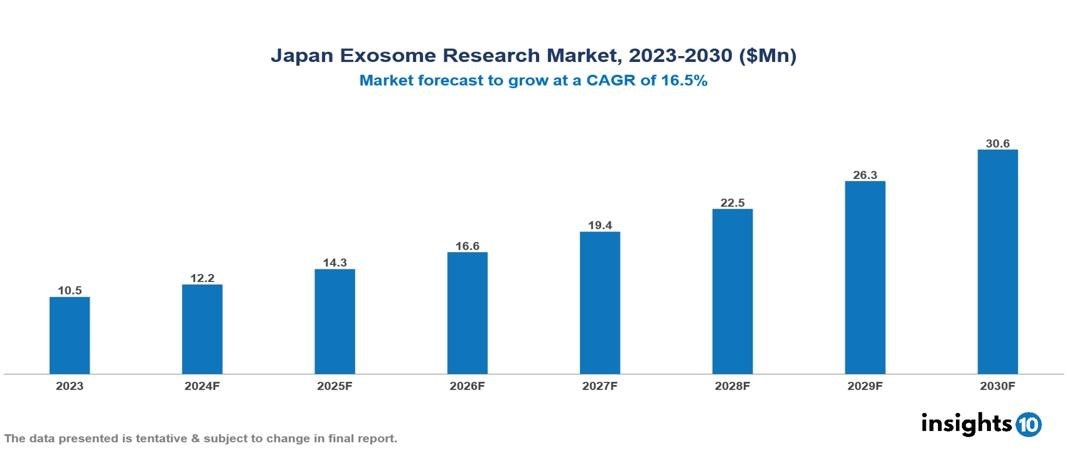
The Japan Exosome Research Market was valued at $10.5 Mn in 2023 and is projected to grow at a CAGR of 16.5% from 2023 to 2023, to $30.6 Mn by 2030. The key drivers of this industry are increasing prevalence and incidences of cancer and various auto-immune diseases, rising healthcare expenditure for better health services, growing R&D activities associated with exosome research, technological advancements in exosome isolation and analytical procedures, increasing advanced applications of exosomes. The industry is primarily dominated by players such as CRAIF, Toyobo, Kangstem Biotech, Digzyme, Thermo Fisher Scientific among others.
Buy Now

Japan Exosome Research Market Executive Summary
The Japan Exosome Research Market is at around $10.5 Mn in 2023 and is projected to reach $30.6 Mn in 2030, exhibiting a CAGR of 16.5% during the forecast period 2023-2030.
Exosomes are nano-sized extracellular vesicles released by most cell types, playing a crucial role in intercellular communication and the transportation of molecules like proteins, lipids, and nucleic acids. The exosome market has garnered significant attention due to its potential applications across various fields. In therapeutics, exosomes can be used as natural drug delivery vehicles to transport therapeutic molecules to target cells or tissues, potentially improving specificity and reducing side effects. Additionally, the molecular composition of exosomes can serve as biomarkers for various diseases, enabling early detection and monitoring. In the cosmeceuticals sector, exosomes derived from stem cells or other sources are being explored for skin rejuvenation and anti-aging products. Furthermore, exosomes are valuable research tools for studying intercellular communication, disease mechanisms, and developing new therapeutic strategies.
Exosomes have significant therapeutic potential, particularly in the context of COVID-19 and other diseases. They can be used as cell-free alternatives for treating various conditions and tissue regeneration by delivering therapeutic cargo components while avoiding immune rejection and cellular toxicity. Stem cell-derived exosomes are particularly advantageous in harnessing the anti-inflammatory and regenerative abilities of parent cells, making them suitable for engineered treatments for respiratory viral diseases like SARS-CoV-2. Exosomes also have promising potential as a vehicle for drug delivery due to their natural material transportation properties, ability to support intrinsic long-term circulation, and high biocompatibility. These factors make them suitable for delivering a variety of proteins, chemicals, and nucleic acids. Research studies have shown positive results for exosomes as mediators of intercellular communication, potentially delivering functional proteins, mRNA transcripts, and miRNAs to cells throughout the body. Additionally, exosomes derived from certain cell types, such as dendritic and mesenchymal stem cells, have therapeutic properties and are biocompatible and efficient agents against various disorders, including organ injury and conditions like heart, kidney, liver, and lung illnesses. These properties make exosomes a promising therapeutic platform for the treatment of a range of diseases.
The market therefore is driven by significant factors like increasing prevalence and incidences of cancer and various auto-immune diseases, rising healthcare expenditure for better health services, growing R&D activities associated with exosome research, technological advancements in exosome isolation and analytical procedures, and increasing advanced applications of exosomes.
Some of the major players operating in the Japan Exosome Research Market are CRAIF, Toyobo, Kangstem Biotech, Digzyme, Thermo Fisher Scientific among others.
Market Dynamics
Market Drivers
Aging Population and Rising Healthcare Demands: By 2040, it is projected that those aged over 65 will account for 34.8% of the population. Japan has one of the highest life expectancies and a rapidly aging population, leading to an increased demand for advanced diagnostics and targeted therapies. Exosomes hold promise in areas such as early disease detection, personalized medicine, and regenerative therapies, positioning them as potential solutions to address Japan's growing healthcare needs.
Government Support and Funding: The Japanese government has recognized the potential of exosome research and has allocated funding for various initiatives and projects. For instance, the Japan Agency for Medical Research and Development (AMED) has supported exosome-related research in areas like cancer and neurodegenerative diseases, driving innovation and market growth.
Strong Pharmaceutical and Biotechnology Industry: Japan has a well-established and globally recognized pharmaceutical and biotechnology industry, with major players such as Takeda, Astellas, and Daiichi Sankyo. These companies have the resources and expertise to invest in exosome research and development, contributing to market growth and commercialization efforts.
Market Restraints
Regulatory Challenges and Approval Processes: The regulatory landscape for exosome-based products in Japan can be complex and stringent. Navigating the approval processes for exosome-based diagnostics and therapeutics can be time-consuming and challenging, potentially hindering market growth and adoption.
High Costs and Reimbursement Concerns: The production, isolation, and purification of exosomes involve specialized techniques and equipment, leading to high costs. Additionally, gaining appropriate reimbursement and pricing for exosome-based products from healthcare payers in Japan may pose challenges, as these are still considered novel and expensive technologies.
Technical Barriers and Standardization Issues: The isolation and characterization of exosomes can be technically complex, requiring specialized expertise and equipment. Furthermore, the lack of standardized protocols and methodologies for exosome research and analysis can lead to variability in results and hinder comparability across studies and products.
Regulatory Landscape and Reimbursement Scenario
PMDA (Pharmaceuticals and Medical Devices Agency) is Japanese regulatory agency, working together with Ministry of Health, Labour and Welfare. Our obligation is to protect the public health by assuring safety, efficacy and quality of pharmaceuticals and medical devices. PMDA conducts scientific reviews of marketing authorization applications (MAA) for medicinal products and monitors post-marketing safety data. PMDA works with the Ministry of Health, Labour, and Welfare (MHLW) to protect the public health safety of Japan. Within PMDA, the Office of Cellular and Tissue-based Products is responsible for regulating cellular therapy products. Health Insurance Bureau develops ideas and plans on medical insurance systems including health insurance, national health insurance, seamen's insurance and medical care for the elderly to stabilize the medical insurance system for long time so that all people can access medical care without worries in a full-fledged aging society with fewer children in the future.
Competitive Landscape
Key Players
Here are some of the major key players in the Japan Exosomes Research Market:
- CRAIF
- Toyobo
- Kangstem Biotech
- Digzyme
- Thermo Fisher Scientific
- Hitachi
- Fujifilm
- NanoSomix
- Malvern Instruments
- Capricor Therapeutic
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Japan Exosomes Research Market Segmentation
By Products
- Instruments
- Software
- Reagents and Kits
By Applications
- Diagnostics
- Therapeutic
By Indication
- Cancer
- Cardiovascular Disease
- Neurogenerative Disease
- Infectious Disease
- Others
By Key End Users
- Hospitals
- Cancer Institutes
- Diagnostic Centers
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.