Japan Digital Therapeutics Market Analysis
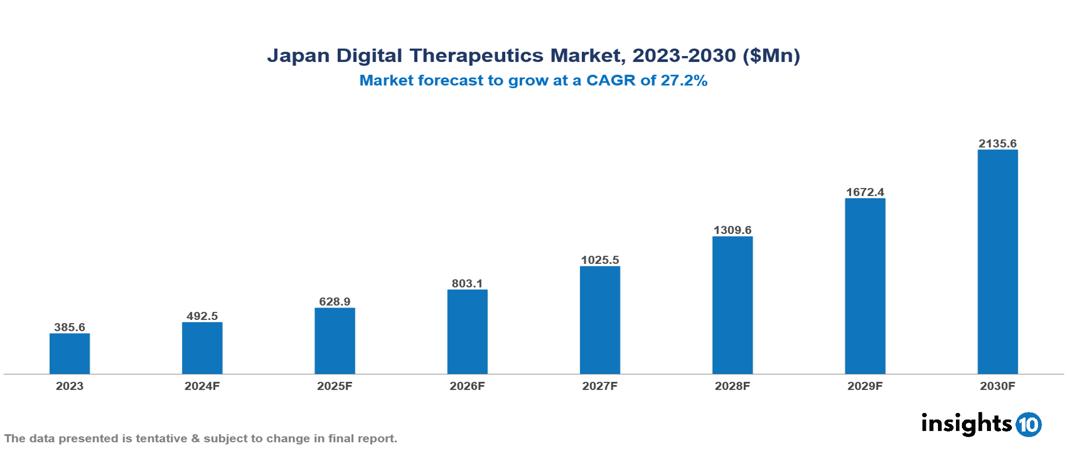
Japan Digital Therapeutics Market is at around $0.4 Bn in 2023 and is projected to reach $2.14 Bn in 2030, exhibiting a CAGR of 27.7 % during the forecast period. The market is growing as a result of rising prevalence of chronic diseases, an aging population, and government initiatives. The market is dominated by key players like CureApp Co., Ltd., Ubie, Inc., Apple Inc., Omada Health Inc., 2Morrow Inc., Livongo Health Inc., WellDoc, Propeller Health, Canary Health, and Noom Inc.
Buy Now

Japan Digital Therapeutics Market Executive Summary
Japan Digital Therapeutics Market is at around $0.4 Bn in 2023 and is projected to reach $2.14 Bn in 2030, exhibiting a CAGR of 27.7 % during the forecast period.
The Japanese market for digital therapeutics is a quickly expanding industry that offers evidence-based treatments for a range of medical diseases by leveraging digital technologies like software, mobile apps, and other digital platforms. This industry is distinguished by the incorporation of cutting-edge technologies to improve overall healthcare administration and patient outcomes. The Digital Therapeutics Market in Japan is promoting breakthroughs in personalized medicine and patient-centric healthcare by utilizing digital solutions to supplement conventional medical techniques.
The market for digital therapeutics in Japan is expanding quickly, propelled by the rising use of digital health technologies. An aging population, an increase in the frequency of chronic illnesses, and government programs supporting digital healthcare are all contributing causes. Businesses in the industry are coming up with new ideas to provide convenient and efficient digital therapy solutions.
The global revenue for digital therapeutics reached $6.2 Bn in 2023, indicating the market's impressive expansion. The fundamental change that modern technology and cost-effective production methods brought forth has resulted in phenomenal growth for this industry. For this reason, the usage of digital treatments in the healthcare sector is expanding. Given that the market's present trend suggests that more changes may be on the horizon, new advancements are anticipated to support the industry's continuous performance.
CureApp creates and distributes digital therapy apps to treat diseases and addictions associated with a lifestyle, filling gaps in care where conventional treatments are insufficient. With a robust product range, regulatory approval, and funding, CureApp is the market leader in Japan for digital therapeutics.
Market Dynamics
Market Growth Drivers:
Growing Prevalence of Chronic Diseases: The need for practical and efficient digital therapeutic solutions is growing due to the increasing incidence of chronic illnesses like diabetes, cardiovascular disease, and mental health issues.
Aging Population: Japan's population is aging and digital therapeutics offer scalable and easily accessible alternatives for controlling medical issues in the elderly population.
Governmental Programs: The use of digital treatments is greatly increased by encouraging government policies and activities that support innovation and digital health. Japan has been making investments in medical technology, and more government backing might spur industry expansion.
Market Restraints:
Regulatory Environment: One potential limitation on the use of digital therapies is the lengthy approval procedure and strict regulatory standards. Ensuring adherence to the regulatory framework is essential for entering and growing the market.
Healthcare System Integration: There may be difficulties in incorporating digital treatments into the current healthcare system. There could be resistance from medical practitioners or challenges integrating new technologies into long-standing practices.
Data Privacy Issues: The adoption of digital therapies may be impacted by worries about patient data security and privacy, given the growing usage of digital solutions in healthcare. Gaining the trust of patients and healthcare providers requires addressing these issues.
Healthcare Policies and Regulatory Landscape
The Pharmaceuticals and Medical Devices Agency (PMDA) is the regulatory agency in Japan that collaborates with the Ministry of Health, Labour, and Welfare. It is necessary to send the NDA dossier and the funds that are required to the PMDA. Once the PMDA has reviewed the NDA dossier, it may ask for more details. The PMDA will authorize medication for sale if they are satisfied with the NDA dossier. Japan has a very thorough and rigorous drug approval process. A New Drug Application (NDA), which is thoroughly examined, must be submitted. The intricacy of drug control in Japan rises as a result of strict regulations limiting the supply of pharmaceuticals to those that fulfill the strictest quality requirements.
Competitive Landscape
Key Players:
- CureApp Co., Ltd.
- Ubie, Inc.
- Apple Inc.
- Omada Health Inc.
- 2Morrow Inc.
- Livongo Health Inc.
- WellDoc
- Propeller Health
- Canary Health
- Noom Inc.
1. Executive Summary
1.1 Digital Health Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Digital Health Policy in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Japan Digital Therapeutics Market Segmentation
By Solution
- Software
- Services
By Deployment
- Cloud-based
- On-premises
By Application
- Therapy
- Diabetes
- Obesity
- CNS
- Respiratory
- CVD
By End-use
- Diagnostic Centres
- Healthcare Players
- Healthcare Research Centres
- Hospitals & Clinics
- Nursing Care Centres
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.











































