Japan Coronary Stents Market Analysis
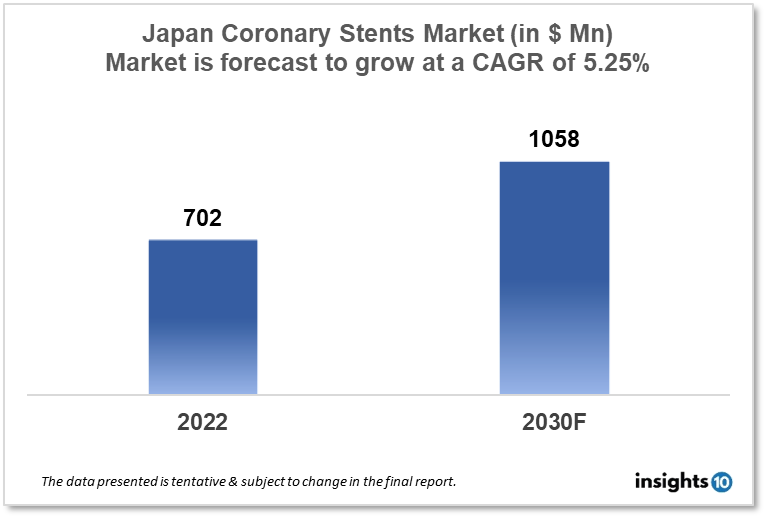
Japan's Coronary Stents Market is expected to witness growth from $702 Mn in 2022 to $1058 Mn in 2030 with a CAGR of 5.25% for the forecasted year 2022-30. The early identification and treatment of coronary artery disease in Japan are projected to be driven by increased access to healthcare and improved heart health awareness, opening up the potential for manufacturers in the coronary stent market. The market is segmented by type, mode of delivery, materials, and end user. Some key players in this market include Terumo Corporation, Fukuda, B. Braun, Abbott Laboratories, Biotronik, Medtronic, GE Healthcare, Boston Scientific, and Philips Healthcare.
Buy Now

Japan Coronary Stents Healthcare Market Executive Analysis
Japan's Coronary Stents Market is expected to witness growth from $702 Mn in 2022 to $1058 Mn in 2030 with a CAGR of 5.25% for the forecasted year 2022-30. The amount spent on healthcare per person in Japan has dramatically increased from $4,360 in 2019, which equaled 10.75% of GDP, to $4,450 in 2022, which would equal 10.74% of GDP. Due to high healthcare spending, the nation has a very developed healthcare infrastructure.
The expected prevalence of coronary artery disease in Japan in 2019 was 4.56% for women and 9.67% for men. The incidence is anticipated to rise to 10.5% among males and 5.5% among women by 2030. In Japan, coronary stents are frequently used to treat coronary artery disease (CAD) and other cardiovascular diseases. Blockages in the coronary arteries that feed blood to the heart muscle are treated with coronary stents. To keep the blocked artery open and allow blood to flow again to the heart, a stent is inserted into it. Restenosis, in which the artery narrows once again due to the development of scar tissue, is a frequent side effect of coronary stent insertion. In Japan, stents that are coated with medications to assist in preventing restenosis are frequently used. When opposed to open surgery, coronary stent installation is a minimally invasive treatment that may be completed through a small incision, which can speed up recovery and minimize problems.
Market Dynamics
Market Growth Drivers
The early identification and treatment of coronary artery disease in Japan are projected to be driven by increased access to healthcare and improved heart health awareness, opening up the potential for manufacturers in the coronary stent market. Japan's coronary stent market is known for being an early user of new technology, therefore producers that create inventive stents with increased safety and efficacy are likely to have a competitive advantage. The Japanese government is devoted to encouraging the adoption of cutting-edge medical equipment and is committed to improving healthcare results. The market can be stimulated by investment and innovation if the government supports medical device makers.
Market Restraints
The market for coronary stents in Japan is quite cutthroat, with both domestic and foreign producers striving for market dominance. To compete in the market, manufacturers must set their products apart from those of their rivals and offer value to consumers. Manufacturers may face difficulties due to Japan's high cost of healthcare since patients and hospitals with limited budgets may pick less expensive coronary stent alternatives. The market for coronary stents in Japan may be impacted by cultural issues as well. For instance, there can be cultural barriers to discussing cardiac issues and receiving medical care, or some patients might favour conventional medicines over medical devices.
Competitive Landscape
Key Players
- Terumo Corporation (JP)
- Fukuda (JP)
- Biotronik
- Boston Scientific
- B. Braun
- Abbott Laboratories
- GE Healthcare
- Medtronic
- Philipps Healthcare
Healthcare Policies and Regulatory Landscape
The Pharmaceuticals and Medical Devices Agency (PMDA) oversees the regulatory framework and healthcare policies in Japan for medical devices, including coronary stents. The primary legislation that controls medical devices in Japan, including coronary stents, is the Japanese Pharmaceutical Affairs Law (PAL). Before marketing their goods in Japan, manufacturers are required by the PAL to have the PMDA's approval. The PMDA mandates that producers carry out post-market surveillance to check on the security and effectiveness of their goods. Manufacturers are required to notify the PMDA of any negative incidents and take the necessary corrective measures as needed.
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Coronary Stents Market Segmentation
By Type (Revenue, USD Billion):
The three main categories of coronary stents are bare-metal, drug-eluting, and bioabsorbable, depending on their nature. The segment for bioabsorbable stents is anticipated to increase at the fastest rate over the forecast period. The benefits that are encouraging the use of bioabsorbable stents include a decrease in problems including thrombosis and inflammation, the cJapanity to restore normal vasomotion, and an improvement in aberrant endothelial function. However, in the upcoming years, the expansion of this market segment is anticipated to be constrained by the high cost of bioabsorbable stents.
- Bare-metal Stents
- Drug-eluting Stents
- Bioabsorbable Stents
By Mode of Delivery (Revenue, USD Billion):
The market is divided into self-expanding and balloon-expandable stents according to delivery method. Because of the high use of these stents, rising research initiatives to advance the technology, and rising regulatory approvals for balloon-expandable stents, the balloon expandable stents segment is anticipated to increase at the greatest CAGR over the projection period.
- Balloon-expandable Stents
- Self-expanding Stents
By Materials (Revenue, USD Billion):
The coronary stent market is divided into metallic (cobalt chromium, platinum chromium, nickel-titanium, and stainless steel) and other stents according to the material. The market for other stents is anticipated to experience the largest CAGR growth during the projection period. The rapid expansion of this market can be ascribed to the increased use of polymers and copolymers in the production of bioabsorbable stents.
- Metallic Stents
- Cobalt-Chromium
- Platinum Chromium
- Nickel Titanium
- Stainless Steel
- Other Stents
By End User (Revenue, USD Billion):
The coronary stent market is divided into hospitals, cardiac centres, and ambulatory surgical centres based on the end user. According to predictions, the hospital sector will control the market in 2016. This is primarily caused by hospitals using a lot of coronary stents.
- Hospitals
- Cardiac Centers
- Ambulatory Surgical Centers
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.









































