Japan Congestive Heart Failure Therapeutics Market Analysis
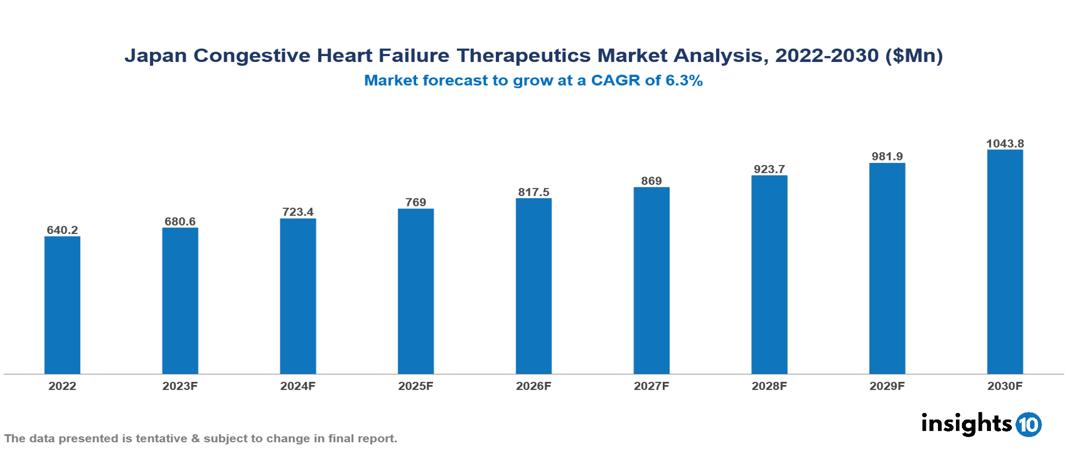
The Japan Congestive Heart Failure Therapeutics Market is anticipated to experience a growth from $640 Mn in 2022 to $1,044 Mn by 2030, with a CAGR of 6.3% during the forecast period of 2022-2030. The primary driving factors include the growing emphasis on quality of life, technological developments in medicine that provide novel therapeutic approaches, and the rising incidence of heart failure as a result of aging populations and sedentary lifestyles in Japan. The Japan Congestive Heart Failure Therapeutics Market encompasses various key players across different therapeutic segments, including Novartis, AstraZeneca, Bayer, Merck, Pfizer, Boehringer Ingelheim, Otsuka, Sumitomo Pharma, Eisai, Meiji Seika Pharma, etc, among various others.
Buy Now

Japan Congestive Heart Failure Therapeutics Market Executive Summary
The Japan Congestive Heart Failure Therapeutics Market is anticipated to experience a growth from $640 Mn in 2022 to $1,044 Mn by 2030, with a CAGR of 6.3% during the forecast period of 2022-2030.
Congestive Heart Failure (CHF) is a chronic medical illness in which the heart is unable to adequately pump blood, resulting in a buildup of fluid in the lungs and surrounding tissues. It is a progressive disorder caused by a variety of underlying conditions, including coronary artery disease, hypertension, and cardiomyopathy. CHF is often categorized into either systolic heart failure, in which the cardiac muscles are unable to contract, or diastolic heart failure, in which they have difficulty relaxing due to stiffness. Treatment for CHF consists of certain lifestyle changes, pharmacological treatment, and, in extreme circumstances, surgical interventions. Diuretics are preferred to minimize fluid retention, ACE inhibitors can decrease blood pressure, and beta-blockers can help in heart function. Dietary modifications, exercise, and weight control are common areas of focus for lifestyle improvements. Technological improvements have had a profound influence on CHF management. Remote monitoring gadgets and wearable technology nowadays allow healthcare providers to monitor patients' vital signs and change treatment strategies remotely. Implantable devices, such as pacemakers and defibrillators, which can help control heart rhythm and improve overall cardiac function are also a treatment of choice in severe cases.
Heart failure is becoming more common in Japan as the country's population ages and its lifestyle becomes more Westernized. Japanese patients with CHF have a worse prognosis than those in Western nations. The hospital in-patient mortality rate owing to CHF in Japan is between 4.7% to 7.5%.
The primary driving factors include the growing emphasis on quality of life, technological developments in medicine that provide novel therapeutic approaches, and the rising incidence of heart failure as a result of aging populations and sedentary lifestyles in Japan.
Among the global pharmaceutical players in the heart failure medication segment, Novartis leads the market in Japan with Entresto and Diovan, dominating certain drug classes. Other players like AstraZeneca, Bayer, and Boehringer Ingelheim offer a diverse portfolio of established Angiotensin Receptor Blockers (ARBs) and Angiotensin-Convertin Enzyme (ACE) inhibitors, providing broader coverage globally. Among local players, Otsuka Pharmaceutical and Sumitomo Dainippon Pharma have a strong market presence with various heart failure medications, while Eisai and Fujifilm Toyama Chemical hold notable positions with established ARBs and combination drugs.
Market Dynamics
Market Growth Drivers
Increasing Focus on Quality of Life: The growing emphasis on elevating the quality of life for individuals with CHF becomes a pivotal driver for increased demand for CHF treatment options. As treatment advancements progress, yielding more favorable patient outcomes and improved quality of life, there is a concurrent rise in the need for innovative therapeutic interventions catering to the holistic well-being of CHF patients.
Innovative Treatment Landscape: The dynamic landscape of CHF treatment experiences a surge in demand driven by the continuous evolution of medical technology. Ongoing advancements give rise to the development of novel drugs, sophisticated devices, and cutting-edge surgical procedures aimed at more efficacious CHF management.
Increased Prevalence: As the global population ages, the risk of heart failure increases significantly. Unhealthy lifestyles, including poor diet, lack of physical activity, and smoking, contribute to the increasing incidence of CVDs, a major risk factor for CHF. Advancements in diagnostic tools and increased awareness about heart health lead to earlier detection and diagnosis of CHF, enabling timely treatment.
Market Restraints
High Cost of Treatment: Innovative medications like ARNI and SGLT2 inhibitors, while offering valuable therapeutic benefits, carry high price tags, creating affordability concerns for patients and healthcare systems. Implantable devices like VADs and ICDs, despite their effectiveness, incur significant costs, limiting accessibility for some patients. The chronic nature of CHF necessitates long-term medication use, putting a strain on individual and national healthcare budgets.
Reimbursement Policies: Japan's National Health Insurance (NHI) has strict cost-effectiveness criteria for drug approval and reimbursement, potentially delaying access to newer, potentially beneficial therapies. Negotiations between pharmaceutical companies and the NHI for drug pricing can be lengthy and complex, creating uncertainty and delaying market entry. Reimbursement for certain CHF therapies, particularly newer technologies, might be limited or unavailable, restricting patient access.
Healthcare System Challenges: Japan's rapidly aging population increases the demand for CHF treatment, putting pressure on limited healthcare resources and personnel. High physician workloads can limit time dedicated to individual consultations and personalized treatment plans for CHF patients. Shortages of healthcare professionals and limited access to specialized care in rural areas can disadvantage CHF patients residing outside major cities.
Healthcare Policies and Regulatory Landscape
The Pharmaceuticals and Medical Devices Agency (PMDA) is a Japanese governmental body tasked with reviewing and regulating drugs and medical devices in Japan. Operating under the Ministry of Health, Labor, and Welfare (MHLW), the PMDA is responsible for activities such as approval reviews, post-market surveillance, and addressing adverse health effects. As the main regulatory authority for medical products in Japan, the PMDA ensures their quality, safety, and efficacy through scientific assessments. The agency provides comprehensive information on its activities, including remote inspection procedures, medical device regulations, and details on job openings and recruitment. Additionally, the PMDA offers resources for foreign manufacturers interested in marketing their products in Japan, including guidelines for accreditation and procedures for classification and approval.
Competitive Landscape
Key Players:
- Novartis
- AstraZeneca
- Bayer
- Merck
- Pfizer
- Boehringer Ingelheim
- Otsuka
- Sumitomo Pharma
- Eisai
- Meiji Seika Pharma
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Japan Congestive Heart Failure Therapeutics Market Segmentation
By Stage of Heart Failure
- Acute Heart Failure
- Chronic Heart Failure
By Drug Class
- ACE Inhibitors
- Beta Blockers
- Angiotensin 2 Receptor Blockers
- Diuretics
- Aldosterone Antagonists
- Others
By Route of Administration
- Oral
- Parenteral
By Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
By End User
- Hospitals
- Speciality Clinics
- Homecare
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.