Japan Clinical Trial Biorepository & Archiving Solutions Market Analysis
Japan Clinical Trial Biorepository & Archiving Solutions Market is projected to grow from $xx Mn in 2023 to $xx Mn by 2030, registering a CAGR of xx% during the forecast period of 2023 - 2030. The market for Clinical Trial Biorepository & Archiving Solutions is growing as a result of expanding fields of precision, personalized, and genomic medicine, increased R&D spending and efforts to make data collection easier by using software like Laboratory Information Management System for the management of laboratory samples. Some of the key players in the global Clinical Trial Biorepository & Archiving Solutions Market include Azenta U.S., Inc., Thermo Fisher Scientific Inc., Precision for Medicine, Inc., Medpace, LabCorp Drug Development, ATCC, Q2 Solutions, Labconnect, Charles River Laboratories, Cell&Co. BioServices, and Brooks Life Science.
Buy Now

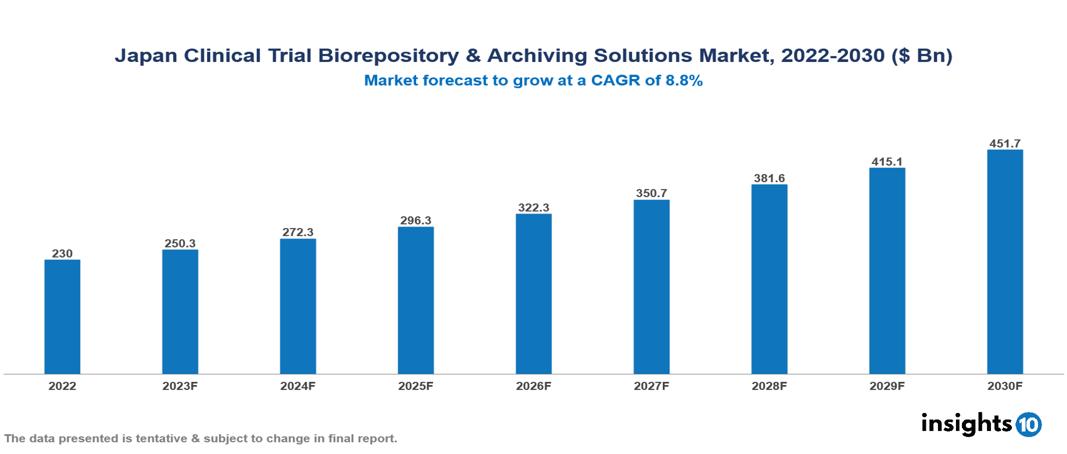
Japan Clinical Trial Biorepository & Archiving Solutions Market Executive Summary
Japan Clinical Trial Biorepository & Archiving Solutions Market is valued at around $230 Bn in 2022 and is projected to reach $451.6 Bn by 2030, exhibiting a CAGR of 8.8% during the forecast period 2023-2030.
The Cholesterol Testing Service Market refers to the market for devices and methods used to test the level of cholesterol in patients. Cholesterol is one of the major risk factors for cardiovascular diseases and cholesterol testing devices helps in early detection of blockage of arteries and cardiovascular diseases. The market includes software and platforms for analyzing and interpreting the findings of blood cholesterol tests, and point-of-care testing to detect the level of cholesterol.
Increasing prevalence of cardiovascular diseases has contributed to the growth of the global market for cholesterol testing service market in recent years. Advances in diagnostic technologies are also driving the market.
There are many players in global cholesterol testing service market but Boston Scientific Corporation, Medtronic, Koninklijke Philips N.V., F. Hoffmann-La Roche Ltd., Abbott, AstraZeneca, LivaNova PLC, BIOTRONIK SE & Co. KG, ACON Laboratories, Inc., Quest Diagnostics Incorporated, Spectra Laboratories, Inc., Eurofins Scientific, and Randox Laboratories Ltd are some of the major players.
Cholesterol level can be measured using a variety of devices and tests, including blood cholesterol tests, non-invasive testing, and point-of-care testing. In blood cholesterol testing total cholesterol test, HDL cholesterol test, LDL cholesterol test, and triglyceride tests are included. All these tests are invasive and venipuncture is needed. Pulse Wave Velocity, ultrasound, and Near-Infrared Spectroscopy are non-invasive methods for cholesterol testing which are being improved with time.
The aging global population, increasing funding for research and development, growing awareness and technological advancements are projected to fuel the market for cholesterol testing services. The market still has many challenges like complex regulatory procedures to enter in market, limited reimbursement coverage and higher cost of non-invasive technologies and instruments.
Market Dynamics
Drivers of Japan Cholesterol Testing Service Market:
Growing Incidence and Prevalence of Cardiovascular Disease: Cardiovascular diseases like heart attack and strokes are increasing with every passing year and high level of cholesterol is major risk factor for these diseases. According to WHO global prevalence of high cholesterol level was 39% in 2022. This is raising the demand for diagnostic methods and technologies that can accurately measure the cholesterol levels.
Aging Population: aging population is increasing with every passing year and people with older age are more likely to suffer from high cholesterol levels and cardiovascular diseases. This is driving the demand for global cholesterol testing service market.
Technological Advancements: New developments like home testing kits and point-of-care testing devices has given a growth to the cholesterol testing market. It has made it easier for individuals to test their cholesterol levels with accuracy, affordability and convenience.
Increasing Awareness: People are becoming more aware about maintenance of heart health which has fueled the demand for cholesterol testing service market. Increased knowledge of risks associated with higher cholesterol level has influenced the people for regular health checkups and cholesterol testing to access their cardiovascular risks.
Restraints of Japan Cholesterol Testing Service Market:
Limited Reimbursement Coverage: Reimbursement coverage for cholesterol testing is very limited in some healthcare systems and it limits the frequency of cholesterol testing which can affect the market growth.
Complex Regulatory Procedure: development and commercialization of cholesterol testing devices are time consuming and requires complex regulatory process. Need for obtaining different necessary certifications, and quality assurance protocols may hinder the growth of global cholesterol testing service market.
Technological Limitations: There has been new developments in technologies but there are still many challenges like accuracy and efficiency of certain testing methods. Reliability for these test kits are low and it an hinder the growth of cholesterol testing market.
Notable Deals in Cholesterol Testing Service Market:
In May 2022, Goodbody Health debuted its primary UK Health "MOT" test. Innovative in-clinic cholesterol and diabetes testing are part of this new test, and results are finalized in minutes. With the help of these tests, doctors can estimate a person's chance of acquiring diabetes or cardiac disease within the next ten years.
In March 2022, Rosalind Franklin University's (RFU) Community treatment Connection and a new collaboration with NorthShore University Health System continued to offer underprivileged people in numerous North Chicago communities’ access to life-saving medical treatment. The Community Investment Fund (CIF) of NorthShore has awarded $680,000 in funding to The Care Coach, a mobile medical unit manned by qualified medical personnel. Blood pressure regulation, diabetes screening, BMI, cholesterol testing, and other complementary medical services are all offered by the mobile medical unit.
Key players
Shimadzu Corporation Thermo Fisher Scientific Inc. Panasonic Healthcare Co., Ltd. PHC Holdings Corporation Hitachi Chemical Co., Ltd. Brooks Automation, Inc. Chart Industries, Inc. Haier Biomedical Micronix Systems Ltd. VWR International, LLC.1. Executive Summary
1.1 Service Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Healthcare Services Market in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Services
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Market Segmentations For Japan Clinical Trial Biorepository & Archiving Solutions Market
By Service:
- Biorepository Services
i. Warehousing & Storage
ii. Transportation
iii. Sample Processing
iv. Others
- Archiving Solution Services
i. Database Indexing Management
ii. Scanning & Destruction
By Product:
- Preclinical
- Clinical
i. Human Tissue
ii. Organs
iii. Stem Cells
iv. Other Biospecimens
By Phase:
- Phase I
- Phase II
- Phase III
- Phase IV
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































