Japan Cardiovascular Diseases Therapeutics Market Analysis
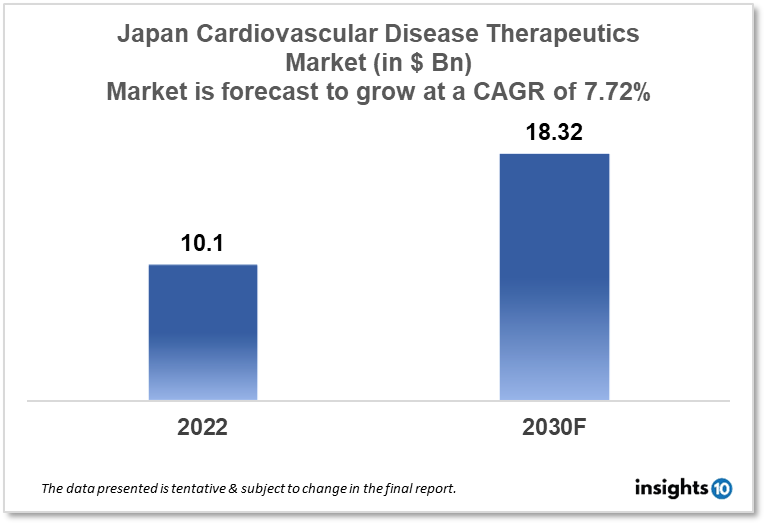
Japan's cardiovascular disease therapeutics market is projected to grow from $10.10 Bn in 2022 to $18.32 Bn in 2030 with a CAGR of 7.72% for the year 2022-30. Japan has the largest elderly population which results in an increased demand for cardiovascular therapeutics in Japan hence driving the growth of the market. The Japan cardiovascular disease therapeutics market is segmented by disease indication, drug type, route of administration, drug classification, mode of purchase, and by end user. Kewpie Corporation, Morunda, and Pfizer are the major players in the Japanese cardiovascular disease therapeutics market.
Buy Now

Japan Cardiovascular Disease Therapeutics Market Executive Analysis
Japan's cardiovascular disease therapeutics market is projected to grow from $10.10 Bn in 2022 to $18.32 Bn in 2030 with a CAGR of 7.72% for the year 2022-30. According to Japan's Federation of Health Insurers, government spending on healthcare for those 75 and elderly is anticipated to reach $229.45 Bn in fiscal 2025, an increase of two-thirds from fiscal 2015. The federation estimates that total healthcare spending will amount to $430 Bn, up from $310 Bn in fiscal 2025.
According to WHO statistics, Japan had two- to three-fold higher mortality from stroke than the US, UK, and France. Over 20% of all medical costs are spent on treating CVD in Japan, making it one of the leading causes of disease burden. Deaths from CVD in the aging population have decreased over the past 50 years, especially in those 80 years of age and older. Even though there are many top-notch medications and medical devices for treating cardiovascular diseases, such as heart disease, there are still a lot of patients globally. Cardiovascular diseases account for the highest yearly medical expenditure in Japan, totaling more than $44 Bn.
Low- and high-density lipoprotein cholesterol (LDL-C and HDL-C), as well as triglycerides, have been the main targets of lipid-modifying therapies for the management of CVD since the advent of HMG-CoA reductase inhibitors (statins) in Japan. Statins, ezetimibe, and fibrates have significant benefits for decreasing the risk of CV events, primarily through lowering circulating levels of LDL-C, according to large randomized outcome studies. Recent studies in Japan have demonstrated the effectiveness of new medicinal targets in reducing LDL-C, including proprotein convertase subtilisin/kexin type (PCSK9) and ATP citrate lyase, which is the target of bempedoic acid. Through lowering LDL-C and perhaps triglycerides, this arsenal of lipid-modifying therapies provides good risk reduction for CVD events.
Market Dynamics
Market Growth Drivers
Japan's population is ageing at one of the fastest rates in the globe, which has increased the incidence of cardiovascular diseases. As a result, there is an increasing need for CVD medicines. The Japanese government has increased healthcare spending in recent years, allowing more people to receive CVD therapeutics. Innovative therapies and medications that are more effective and have fewer side effects have been introduced as a result of the development of new and cutting-edge technologies in the area of CVD therapeutics. The use of novel treatments and medications has been aggressively encouraged by the Japanese government, which has contributed to Japan's cardiovascular disease therapeutics market growth.
Market Restraints
The Japanese regulatory framework for the approval of CVD therapeutics is quite stringent, which may cause a delay in the release of new medications and treatments. Alternative treatments, like non-pharmacological interventions and conventional medications, can compete with CVD therapeutics and restrict their potential for development hence slowing down the growth of Japan's cardiovascular disease therapeutics market.
Competitive Landscape
Key Players
- Myoridge (JPN)
- Nobelpharma (JPN)
- Rekumedo (JPN)
- Kewpie Corporation (JPN)
- Morunda (JPN)
- Pfizer
- Lupin
- GSK
- Glenmark Pharmaceuticals
- Capricor Therapeutics
- Zensun
Healthcare Policies and Regulatory Landscape
All Japanese citizens are required to have health insurance, which covers a broad range of healthcare services, including those related to CVD. Low-income people receive government assistance with their health insurance premiums, ensuring their access to medical treatment. For therapies and treatments for CVD, the Japanese government has adopted a reimbursement policy. NHI (National Health Insurance) and MHLW (Ministry of Health, Labour, and Welfare) designations make up the two divisions of the reimbursement system. Patients will have access to reasonably priced CVD treatments attributable to this method. For people with CVD, the Japanese government has instituted a disease management program. To successfully manage their condition, patients have access to coordinated treatment through this program, including routine check-ups, education, and counselling. The Japanese government encourages regular health screening for the early detection of CVD. Based on age and gender, these screenings are given to all residents regularly.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Cardiovascular Disease Therapeutics Segmentation
By Disease Indication (Revenue, USD Billion):
- Hypertension
- Coronary Artery Disease
- Hyperlipidaemia
- Arrhythmia
- Others
By Drug Type (Revenue, USD Billion):
- Antihypertensive
- Anticoagulants
- Antihyperlipidemic
- Antiplatelet Drugs
- Others
By Route of Administration (Revenue, USD Billion):
- Oral
- Parenteral
- Others
By Drug Classification (Revenue, USD Billion):
- Branded Drugs
- Generic Drugs
By Mode of Purchase (Revenue, USD Billion):
- Prescription-Based Drugs
- Over-The-Counter Drugs
By End Users (Revenue, USD Billion):
- Hospital Pharmacies
- Online Pharmacies
- Retail Pharmacies
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































