Japan Brugada Syndrome Market Analysis
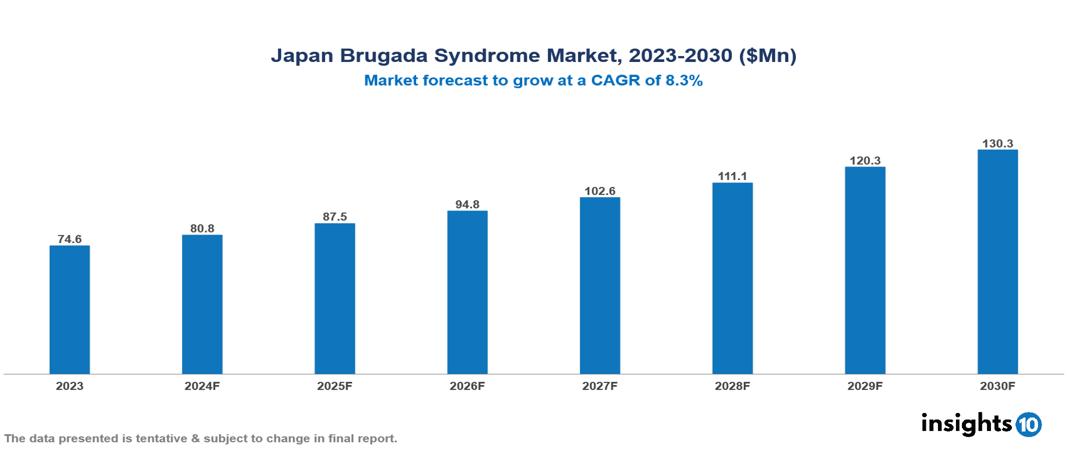
The Japan Brugada Syndrome Market was valued at $74.6 Mn in 2023 and is predicted to grow at a CAGR of 8.3% from 2023 to 2030, to $130.3 Mn by 2030. The key drivers of the market include growing prevalence of cardiovascular diseases, aging population, and advancements in genetic testing. The prominent players of the Japan Brugada Syndrome Market are Takeda Pharmaceuticals, Astellas, Canon Medical Systems Corporation, Terumo, Nipro, and Otsuka, among others.
Buy Now

Japan Brugada Syndrome Market Executive Summary
The Japan Brugada Syndrome Market is at around $74.6 Mn in 2023 and is projected to reach $130.3 Mn in 2030, exhibiting a CAGR of 8.3% during the forecast period.
Brugada syndrome is a rare, but potentially threatening, genetic condition that causes abnormal electrical activity in the heart, leading to an increased risk of sudden cardiac death. People with Brugada syndrome have an increased risk of irregular heart rhythms beginning in the lower chambers of the heart, i.e., the ventricles. Common signs and symptoms associated with Brugada Syndrome include dizziness, fainting, gasping and laboured breathing, particularly at night, irregular heartbeats or palpitations, extremely fast and chaotic heartbeat, and seizures. The risk factors for Brugada syndrome include family history of Brugada syndrome, being male, race, and fever.
Cardiovascular diseases (CVDs), such as heart disease and stroke, have a significant impact on life expectancy, healthy life expectancy, and medical costs in Japan and have a weighted prevalence of 37.3%. The Japan Brugada Syndrome Market is driven by significant factors such as growing prevalence of cardiovascular diseases, aging population, and advancements in genetic testing. However, high cost of treatment, side effects and complications of treatment, and stringent regulations restrict the growth and potential of the market.
The major players of the Japan Brugada Syndrome Market are Takeda Pharmaceuticals, Astellas, Canon Medical Systems Corporation, Terumo, Nipro, and Otsuka, among others.
Market Dynamics
Market Growth Drivers
Growing Prevalence of Cardiovascular Diseases: The prevalence of the most common subtypes of CVD was: carotid artery disease 20.5%, coronary heart disease 11.9%, and cerebrovascular disease 10.4%. As cardiovascular conditions become more common due to aging populations, lifestyle changes, and higher rates of hypertension and diabetes, there is a heightened awareness and focus on genetic disorders like Brugada syndrome. This growing prevalence encourages greater investments in diagnostic tools, research, and advanced treatments for Brugada syndrome, as healthcare systems strive to address the broader spectrum of cardiovascular health issues. Consequently, the demand for effective management and therapeutic options for Brugada syndrome is anticipated to rise, thereby fueling market growth.
Aging Population: When it comes to having the most significant aging population, Japan takes the lead. 28.2% of Japan’s population is 65 years old or older, which equates to around 36.23 Mn people. 34% of those people are between 75 and 84, and 16% are over 85. With rising life expectancies, the occurrence of age-related heart conditions such as Brugada syndrome is anticipated to increase. This demographic change is likely to boost demand for diagnostic tools, therapeutic interventions, and patient monitoring devices. Additionally, the expanding elderly population require enhanced healthcare infrastructure and specialized facilities to manage Brugada syndrome, further driving market growth.
Advancements in Genetic Testing: Advancements in genetic testing in Japan are playing a crucial role in the growth of the Brugada syndrome market. Japan's leading position in genetic research is benefiting rare diseases such as Brugada syndrome through enhanced testing capabilities that enable earlier and more precise diagnoses. This leads to timely interventions and the prevention of severe arrhythmias. Consequently, there is a rising demand for genetic testing services, along with diagnostic tools, therapeutic options, and patient monitoring devices. This emphasis on genetic testing is expanding the market by identifying a larger patient base and promoting personalized treatment strategies.
Market Restraints
High Cost of Treatment: The high cost of Brugada syndrome treatment in Japan represents a major obstacle to market growth. Despite progress in diagnostics and therapies, the significant expenses associated with implantable cardioverter-defibrillators (ICDs), antiarrhythmic medications, and other treatment options create a substantial financial burden. This cost limits access to care for many patients, especially those lacking comprehensive insurance, which impedes market expansion.
Side Effects and Complications of Treatments: Side effects and complications from Brugada syndrome treatments can significantly restrain market growth in Japan. Although implantable cardioverter-defibrillators (ICDs) and antiarrhythmic medications are essential for managing the condition, they can result in complications such as device-related infections, arrhythmias, and other adverse reactions. Concerns about these potential side effects lead patients to avoid treatment or not follow prescribed medication regimens, impeding market expansion.
Stringent Regulations: Japan's strict regulatory requirements for approving diagnostic tests and drugs pose a considerable challenge for the Brugada syndrome market. While these rigorous standards are designed to ensure patient safety, they also lengthen the time and resources needed for product development and approval. This process can delay market entry, restrict the availability of innovative diagnostic tools and treatments, and impede the overall growth of the Brugada syndrome market in Japan.
Regulatory Landscape and Reimbursement Scenario
The PMDA (Pharmaceuticals and Medical Devices Agency) is Japanese regulatory agency, working together with Ministry of Health, Labour and Welfare (MHLW). Their core purpose is to ensure the safety, quality, and efficacy of the pharmaceuticals and medical devices in the Japan.
The PMDA conducts thorough review and evaluation of the new drugs based on safety, quality, and efficacy seeking marketing authorization in Japan. This minimizes the risk of patients experiencing adverse effects from medications or faulty medical devices. The PMDA’s responsibility extends beyond granting market authorization and includes post-marketing surveillance for reporting any adverse drug reactions. As a founding country, Japan is an important member in the development and application of ICH (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use) guidelines. The PMDA regularly participates in ICH meetings and contributes to establishing harmonized standards for the pharmaceutical industry. Japanese pharmaceutical companies can expedite the registration of their products in Japan and other ICH member nations by following ICH rules. This makes it easier for innovative drugs to be marketed internationally more quickly.
The reimbursement system in Japan plays a crucial role in balancing access to care with affordability. The majority of Japanese citizens are covered by the National Health Insurance (NHI), a government-mandated insurance scheme. For inpatient and outpatient services at approved medical facilities, NHI reimburses a significant percentage of medical expenses. However, deductibles and copayments may be necessary based on the service, the patient’s age, and income. Government subsidies are provided for certain services, such long-term care and some chronic conditions, the government offers extra funding. In comparison to NHI, voluntary private insurance policies may provide more coverage and better reimbursement rates. These plans can pay for expenses that NHI does not cover, such as sophisticated procedures and diagnostics, shorter waiting times for some medical procedures, and individual hospital rooms.
Competitive Landscape
Key Players
Here are some of the major key players in the Japan Brugada Syndrome Market:
- Takeda Pharmaceuticals
- Astellas Pharma
- Canon Medical Systems Corporation
- Terumo
- Nipro
- Otsuka
- Asahi Kasei
- Daiichi Sankyo
- GE Healthcare
- Edwards Lifesciences
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Japan Brugada Syndrome Market Segmentation
By Diagnosis
- Electrocardiogram
- Electrophysiology (Ep) Test
- Genetic Testing
By Treatment
- Implantable Cardioverter-Defibrillator
- Drug Therapy
By End User
- Hospitals
- Clinics
- Diagnostic Centres
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































